Heteroatom-containing novel high B-ring berberine analogues and C-H activation synthesis method thereof
A heteroatom and berberine technology, applied in the field of synthesis of new high-B-ring berberine analogs and their C-H activation, can solve the problems of difficult discovery of candidate compounds, few chemical transformations of berberine, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
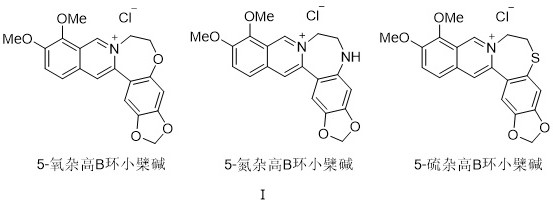
[0018] Implementation example 1: Synthesis of 5-oxahomo-B ring berberine (Ber-1)
[0019]
[0020]
[0021] Starting with 2,3-dimethoxybenzylamine and 3,4-methylenedioxybromobenzene, acylation, iodolation, Sonogashira coupling, C–H activation / cyclization, nucleophilic substitution, and cyclization and other steps to obtain the target compound 5-oxahomoB ring berberine (Ber-1).
[0022] (1) Synthesis of compound 3:
[0023] Add 2,3-dimethoxybenzylamine 1 (4 mmol) and 2-pyridinecarboxylic acid 2 (4 mmol, 1.0 equiv.) into a dry 50 mL two-necked flask, replace with argon 3 times, inject DCM ( 20 mL) was dissolved, and the reaction solution was pre-cooled at -10 °C for 5 minutes. Join Et 3 N (8 mmol, 2.0 equiv.), after stirring evenly, slowly drop POCl 3 (8 mmol, 2.0 equiv.). After dropping, react at -10°C for 5 hours and then move to room temperature overnight. After the completion of the TLC monitoring reaction, it was transferred to a separatory funnel, followed by s...
Embodiment 2
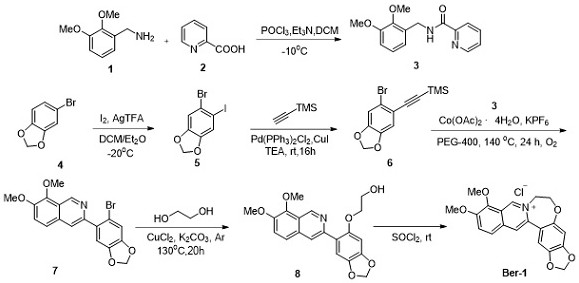
[0040] Implementation example 2: Synthesis of 5-azahomo-B ring berberine (Ber-2)
[0041]
[0042] As shown in the above synthetic route, the target compound 5-azahomoB-ring berberine (Ber-2) was obtained by using the key intermediate 7 as a raw material through nucleophilic substitution, cyclization and other steps.
[0043] (1) Synthesis of Compound 9:
[0044] Compound 7 (0.26 mmol), CuCl (0.026 mmol, 0.1 equiv.), KOH (0.52 mmol, 2.0 equiv.) and 4.0 mL of aminoethanol were sequentially added into a 10 mL round bottom flask. The reaction solution was placed at 90°C for 8 hours. After the completion of the reaction monitored by TLC, compound 9 was separated and purified by direct column chromatography (eluent: petroleum ether / ethyl acetate = 2 / 1), with a yield of 45%.
[0045] Compound 9 is a yellow solid, 1 H NMR (400 MHz, DMSO- d 6 ) δ 9.36 (s, 1H), 8.06 –8.03 (m, 2H), 7.74 (s, 2H), 7.22 (s, 1H), 6.45 (s, 1H), 5.94 (s, 2H), 4.78(t, J = 5.2 Hz, 1H), 4.00 (s, 3H), 3...
Embodiment 3
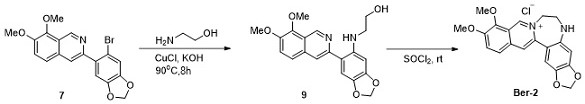
[0049] Example 3: Synthesis of 5-thiahomoB-ring berberine (Ber-3)
[0050]
[0051] As shown in the above synthetic route, the target compound 5-thiahomoB-ring berberine (Ber-3) was obtained by using the key intermediate 7 as a raw material through nucleophilic substitution, cyclization and other steps.
[0052] (1) Synthesis of compound 10:
[0053] Compound 7 (0.13 mmol), CuO (0.26 mmol, 2.0 equiv.), mercaptoethanol (1.3 mmol, 10.0 equiv.) and 2.0 mL of dioxane were sequentially added to a 15 mL sealed tube. The reaction solution was reacted at 130° C. for 24 hours. After the completion of the reaction monitored by TLC, water and ethyl acetate were added for extraction, and the organic layer was concentrated and then column chromatographed (eluent: petroleum ether / ethyl acetate = 2 / 1) to obtain compound 10 with a yield of 85%.
[0054] Compound 10 is a yellow solid, 1 H NMR (400 MHz, DMSO- d 6 ) δ 9.40 (s, 1H), 7.87 –7.77 (m, 3H), 7.19 (s, 1H), 7.08 (s, 1H), 6.10 (s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com