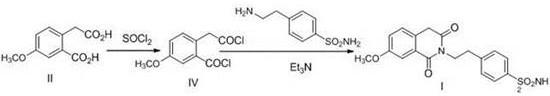
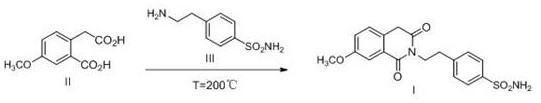
Preparation method of gliquidone intermediate
A technology for gliquidone and intermediates, which is applied in the field of preparation of gliquidone intermediates, can solve problems such as increased operating procedures, unfriendliness, and increased consumption of thionyl chloride and triethylamine, so as to reduce consumption costs, Increase the conversion rate and reduce the effect of waste gas treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 210 g of compound II, 200 g of compound III and 1200 ml of water were added to the autoclave, sealed, stirred, heated to 160° C. to react for 8 hours, sampled through a sampling valve, 0.17% of the residual amount of compound IIII was detected by HPLC, the heating was stopped, and the stirring was continued. When cooled to 25°C, gradually open the exhaust valve. After the air pressure was equilibrated, the feed liquid was directly filtered to obtain a pale yellow crude product, washed with water, dried at 60° C. to obtain 359 g of Intermediate I, the yield was 96.0%, detected by HPLC, and the area-normalized purity was 99.7%.
Embodiment 2
[0035] 210 g of compound II, 196 g of compound III and 1200 ml of n-propanol were added to the autoclave, sealed, stirred, heated to 155° C. to react for 8 hours, sampled through a sampling valve, 0.86% of the residual amount of compound IIII was detected by HPLC, the heating was stopped, and the stirring was continued. When cooled to 30°C, gradually open the exhaust valve. After air pressure equilibration, the feed liquid was directly filtered to obtain an off-white crude product, washed with n-propanol, and dried at 60° C. to obtain 337 g of Intermediate I in a yield of 90.1%, detected by HPLC, and the area-normalized purity was 99.3%.
Embodiment 3
[0037] 210 g of Compound II, 210 g of Compound III and 1300 ml of acetonitrile were added to the autoclave, sealed, stirred, heated to 150° C. to react for 8 hours, sampled through a sampling valve, 0.06% of the residual amount of Compound IIII was detected by HPLC, the heating was stopped, and the stirring was continued. When cooled to 20°C, gradually open the exhaust valve. After air pressure equilibration, the feed liquid was directly filtered to obtain a white product, washed with acetonitrile, dried at 60° C. to obtain 347 g of Intermediate I, the yield was 92.8%, detected by HPLC, and the area-normalized purity was 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com