Bispecific antibody with double Her2 sites for tumor immunotherapy
A bispecific antibody and site technology, applied in the field of host cells, can solve problems such as poor internalization and drug resistance, and achieve the effects of improving binding capacity, enhancing permeability, and improving spatial flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1. Design and purification of bispecific antibody Bp-Bs and its control Bi-Bs
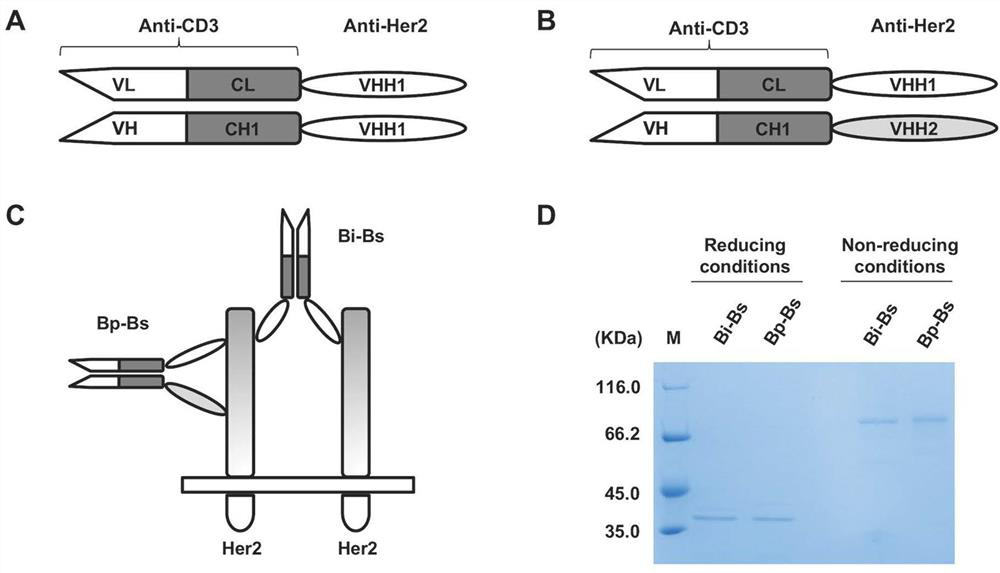
[0059] The structures of the bivalent anti-Her2 bispecific antibody (Bi-Bs) and the bispecific antibody Bp-Bs binding to Her2 double sites, respectively figure 1 A and 1B are shown. DNA shuffling and ligation techniques were used to clone the respective genes. Wherein, Bi-Bs: single chain domain anti-Her2 VHH1 (SEQ ID NO.1, GenBank: JX047590.1; Even-Desrumeaux, K., P. Fourquet, V. Secq, D. Baty and P. Chames ( 2012). "Single-domain antibodies: a versatile and rich source of binders for breast cancer diagnostic approaches." MolBiosyst 8(9): 2385-2394.) VH-CH1 ligated to anti-CD3 UCHT1 clone (with linker: (GGGGS) 3 )) and the C-terminus of VL-CL; and for Bp-Bs: replace the anti-Her2 VHH1 at VH-CH1 of Bi-Bs with another anti-Her2 VHH2 (SEQ ID NO.2; Wu, X ., S. Chen, L. Lin, J. Liu, Y. Wang, Y. Li, et al.(2018). "A Single Domain-Based Anti-Her2 Antibody Has Potent Antitumor Activities...
Embodiment 2
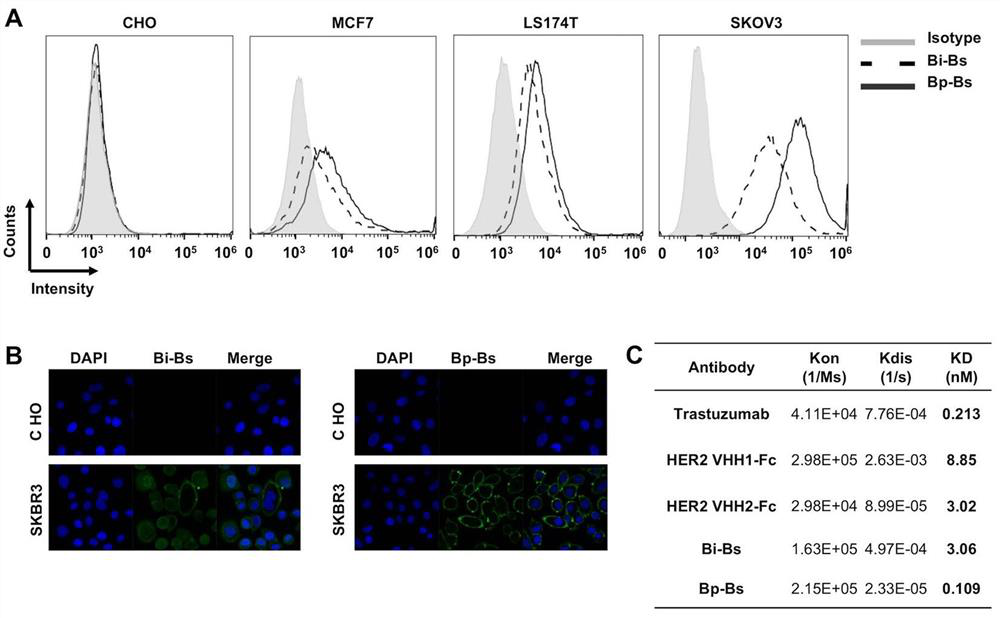
[0060] Example 2. Binding properties of the Bp-Bs antibody
[0061] experimental method:
[0062] Cell lines: CHO, MCF7, LS174T, SKOV3, SKBR3 cells are all from the cell bank of the Type Culture Collection Committee of the Chinese Academy of Sciences; the medium used for cell culture, fetal bovine serum, trypsin, penicillin-streptomycin antibiotic mixture and other additives are purchased from from Gibco; consumables used for cell culture were purchased from Corning Costar. All cell lines were incubated at 37°C, 5% CO 2 DMEM (for MCF7, SKBR-3 and SKOV3) or RPMI-1640 (Thermo, China) containing 10% HI fetal bovine serum (Thermo, USA) and 1% penicillin / streptomycin (Hyclone) (for MCF7, SKBR-3 and SKOV3) (with cultured in LS174T and CHO).
[0063]Affinity Determination: The affinity of the anti-Her2 antibody to the extracellular region of Her2 protein was determined using an OctetQKe instrument (Pall Life Sciences). Briefly, Fc-tagged human Her2 (AcroBiosystem, Cat. No. HE2-H5...
Embodiment 3
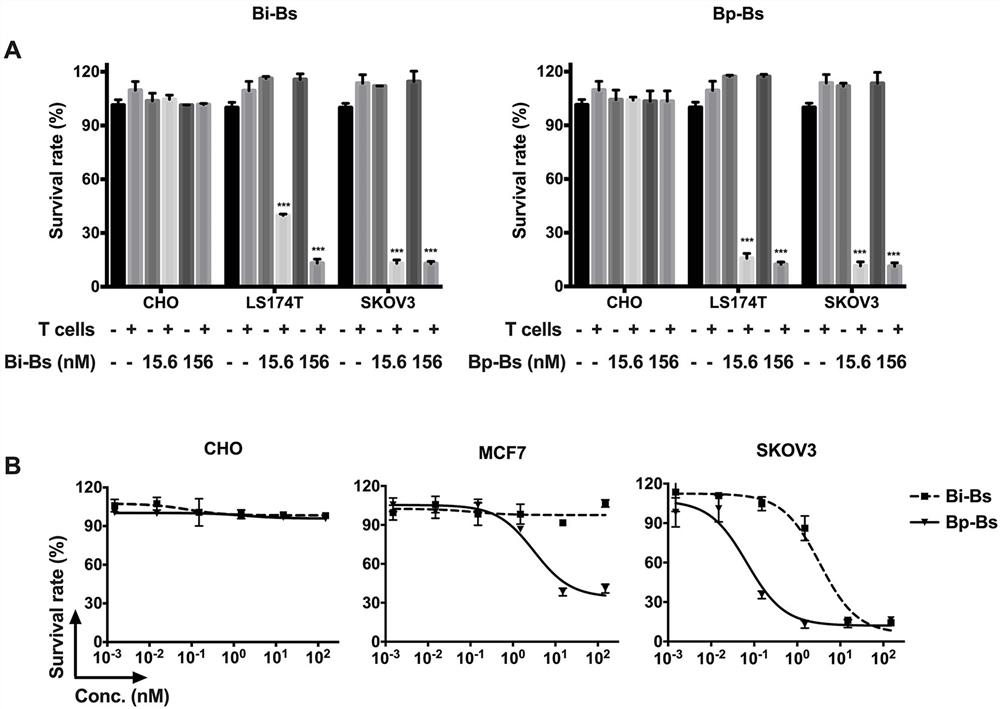
[0070] Example 3. Bp-Bs antibody induces T cell-mediated cytotoxicity
[0071] experimental method:
[0072] To measure bispecific antibody cytotoxicity in vitro, human peripheral blood mononuclear cells (PBMCs) were freshly prepared from fresh donated blood using Ficoll-Plaque Plus (GE health) gradient centrifugation. Human peripheral blood was collected from healthy volunteers with written permission. Then use EasySep TM Human CD3 Positive Selection Kit (StemcellTechnologies, Inc., Vancouver, BC, Canada) Human CD3 was isolated from PBMC according to the manufacturer's instructions + T cells. Cytotoxicity assays were performed as previously described (Li, L., P. He, C. Zhou, L. Jing, B. Dong, S. Chen, et al. (2015). "A novel bispecific antibody, S- Fab, induces potent cancer cell killing." J Immunother 38(9): 350-356.). Briefly, SKOV3, MCF7, LS174T or CHO cancer cells were trypsinized and seeded at a density of 5000 cells / well in 96-well tissue culture plates as target ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com