Retinochrome degeneration macaque model construction method based on in-vivo gene knockout
A retinal pigment and gene technology, applied in the field of molecular biology, can solve the problems of low number of offspring, limitations of transgenic macaque RP models, obstacles to RP disease research and treatment development, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1. In vivo gene transduction of macaque retinal photoreceptor cells
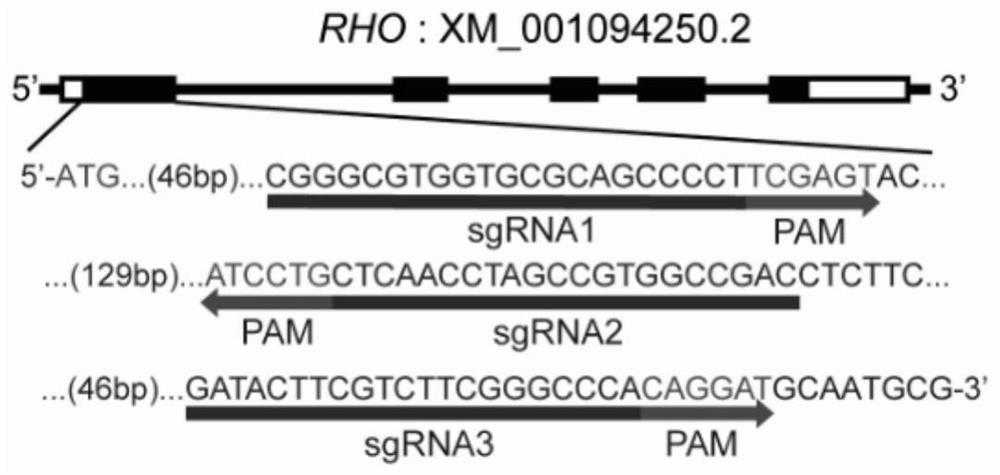
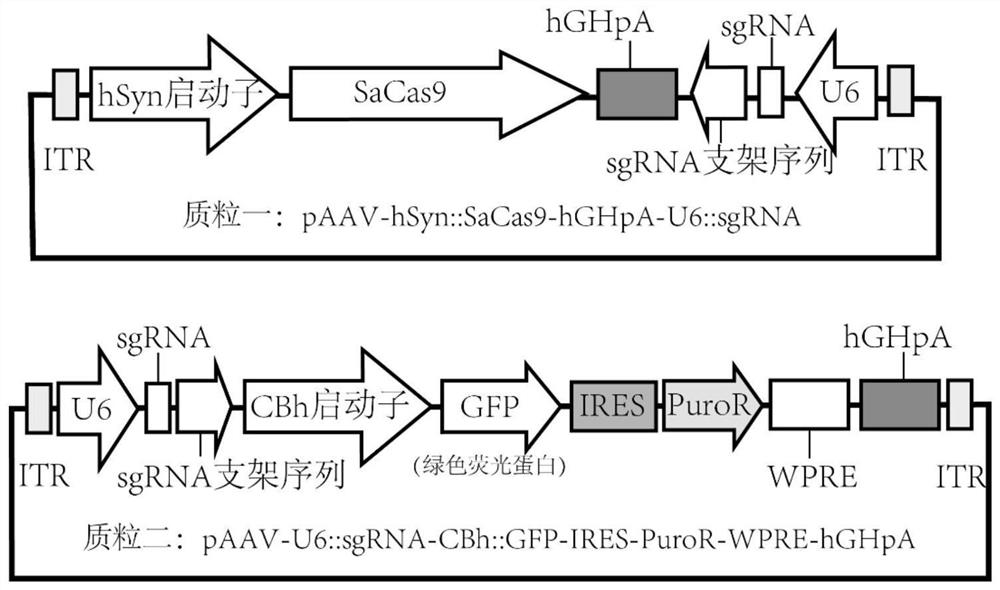
[0055] In this example, taking the RHO gene as an example, we designed three sgRNAs targeting the rhesus monkey RHO gene, such as figure 1 As shown, the specific sequences of sgRNA1-3 are respectively SEQ ID NO:3, SEQ ID NO:4, and SEQ ID NO:5. Subsequently, three sgRNAs were constructed in figure 2 After the U6 promoter in the plasmid-AAV vector, the subsequent packaged AAV can express the Cas9 gene editing enzyme and sgRNAs at the same time. This vector is designed to be transformed from Zhangfeng pX602 plasmid. Specifically: the TBG promoter in the Addgene plasmid #61593 (pX602) was digested with Xba1 / Age1 and replaced with the hSyn promoter. The transformed plasmid is as follows figure 2 As shown in plasmid one, its full vector sequence is SEQ ID NO:1. Taking sgRNA1 as an example, two short DNA sequences CACCGCGGGCGTGGTGCGCAGCCCCT (SEQ ID NO: 8) and AAACAGGGGCTGCGCACCACGCCCGC (SEQ ID ...
Embodiment 2
[0063] Example 2. Rhesus monkey retinal photoreceptor cell RHO knockout detection
[0064] In Example 2, as in Example 1, we packaged the AAV virus and completed the subretinal injection of macaques. At the same time, we injected AAV-GFP and SaCas9 into the left eye of macaques, that is, this group of viruses does not contain sgRNA, so The group that cannot target RHO and knock it out is called the control virus group; while we injected AAV-GFP and SaCas9 / sgRNAs into the right eye, this group of viruses can effectively target RHO and knock it out, which we call the experimental virus group.
[0065] Rhesus monkeys were sacrificed and sampled three months after injection. After surgery, we obtained retinal samples from rhesus monkeys. The whole retinal genome was extracted with a genome extraction kit (Qiagen), and the primers CATTCTTGGGTGGGAGCAGA (SEQ ID NO: 14) and CAAGGTAGCGTTCAGAGCCA (SEQ ID NO: :15) Perform PCR (NEB, Q5) to specifically amplify the first exon of RHO, and p...
Embodiment 3
[0066] Example 3. Photoreceptor cell degeneration (injection for 4 months)
[0067] Same as in Example 2, we sacrificed samples from rhesus monkeys injected with virus for 4 months, and carried out frozen sections (14 microns) of rhesus monkey retinas after virus injection, immunohistochemical staining with antibody RHO (sigma), and using Laser confocal microscope for microscopic observation. Immunohistochemistry is a common biological experiment method, which will not be repeated here.
[0068] Such as Figure 5 As shown, GFP is the green fluorescent protein expressed by AAV-GFP, and it can be seen that it is distributed in the photoreceptor cells in the outer nuclear layer, that is, our method achieves efficient in vivo gene transduction of rhesus monkey retina photoreceptor cells. At the same time, in the RHO immunohistochemical display, it can be seen that the RHO immunofluorescence signal is significantly reduced, and the structure of the outer and inner segments of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com