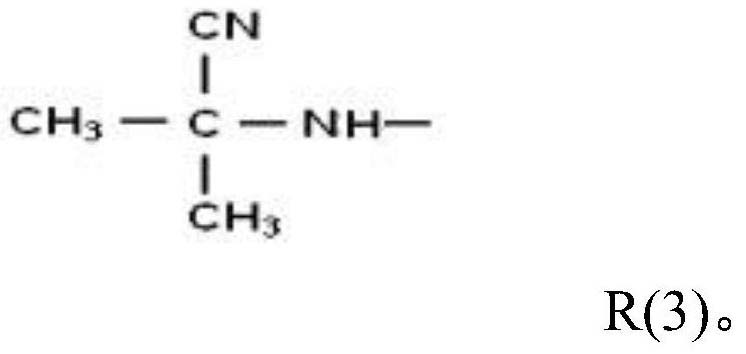
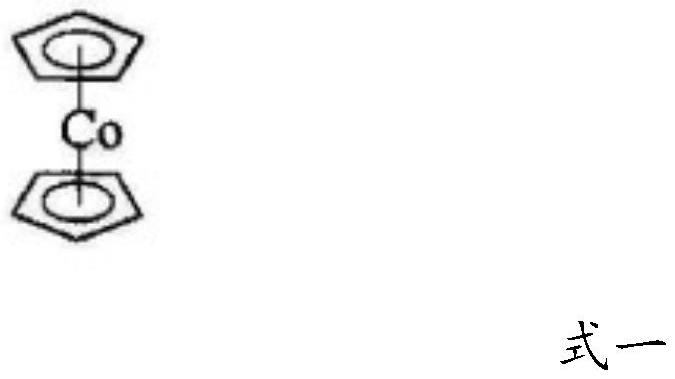Betahistine synthesis method
A synthetic method, the technology of betahistine, which is applied in the field of betahistine synthesis, can solve the problems that the product yield cannot reach this level, strict production conditions, poor control, etc., and achieve high product yield and low reaction temperature. Low, good safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis method of betahistine provided in this embodiment comprises the following steps: 100kg of 3-methylaminopropionitrile, 100kg of xylene, 0.3kg of catalyst cyclopentadienyl cobalt are put into a 1000L autoclave, Next, replace it with high-purity nitrogen three times, pass in purified and dry acetylene gas to 0.1MPa, heat up to 70°C, then continue to pass in acetylene gas, keep the temperature at 85°C, and control the pressure at about 0.6MPa. When the acetylene is contained, the pressure in the reactor rises sharply. At this time, the reaction ends, and the injection of acetylene gas is stopped. The pressure drops to 0.08MPa, and the temperature drops to 40°C. The analysis can obtain a content of 82% betahistine crude product and 2 % of by-product benzene and about 15% of unreacted raw materials and other impurities, the yield is greater than 80%.
Embodiment 2
[0050] The synthesis method of betahistine provided in this example comprises the following steps: 100kg of 3-methylaminopropionitrile, 600kg of benzene, 5kg of catalyst cyclopentadienyl cobalt are put into a 1000L autoclave, and the mixture is placed under a negative pressure of 0.09MPa. , replace with high-purity nitrogen three times, pass in purified and dry acetylene gas to 0.1MPa, heat to 65°C, then continue to pass in acetylene gas, keep the temperature at 95°C, and control the pressure at about 0.8MPa, when 63kg of During acetylene, the pressure in the reactor rises sharply. At this time, the reaction is over, stop injecting acetylene gas, the pressure drops to 0.08MPa, and the temperature drops to 40°C. The content of the crude product of betahistine is 88% and 2% is analyzed except for the solvent. The by-product benzene and about 10% unreacted raw materials and other impurities, the yield is greater than 85%.
Embodiment 3
[0052] The synthetic method of betahistine provided by the present embodiment comprises the following steps: drop into 100kg 3-methylaminopropionitrile, 250kg of toluene, 2kg of catalyst a in a 1000L autoclave Cobalt and azobisisobutyronitrile are reacted in 5 times the molar amount of tetrahydrofuran), and under the negative pressure of 0.09MPa, replace with high-purity nitrogen three times, pass in purified and dry acetylene gas to 0.1MPa, and heat to 65°C , and then continue to feed acetylene gas, keep the temperature at 90°C, and control the pressure at about 0.9MPa. When 65kg of acetylene is fed into the reactor, the pressure in the reactor rises sharply. At this time, the reaction is over, stop injecting acetylene gas, and the pressure drops to 0.08MPa , the temperature was lowered to 40°C, and the analysis could obtain 96% betahistine crude product and 2% by-product benzene and 2% unreacted raw materials and other impurities except the solvent, and the yield was greater ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com