A kind of linear azobenzene polymer containing hydrogen bond and its preparation method and application
A linear azobenzene and polymer technology, applied in the field of polymer materials, can solve problems such as unfavorable large-scale industrial production, complex material synthesis, inability to reprocess, etc., and achieves low reaction environment and equipment requirements, simple synthesis steps, The effect of simplifying the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
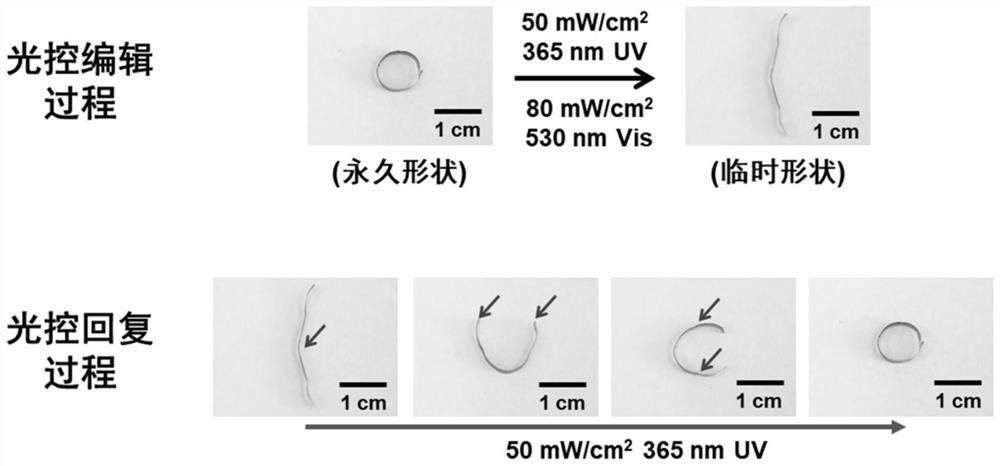
Image
Examples
Embodiment 1
[0072] Example 1: Synthesis of azobenzenediol AB1
[0073] Taking the 4,4'-dihydroxyazobenzene that is 5 parts by weight, the 6-bromo-1-hexanol that is 10 parts by weight, the potassium carbonate or potassium hydroxide that is 20 parts by weight, the weight parts are 0.1 part of potassium iodide, four raw materials were dissolved in 200 parts by weight of 2-butanone, N,N-dimethylformamide or N,N-dimethylacetamide, and the reaction was stirred at 80°C 12h, the solution was precipitated in water and recrystallized twice with absolute ethanol. The obtained solid was vacuum-dried at 40 °C for 12 h to obtain high-purity azobenzenediol AB1. The reaction equation for monomer AB1 is:
[0074]
[0075] The infrared spectrum and hydrogen nuclear magnetic resonance spectrum data of monomer AB1 are as follows:
[0076] FT-IR(KBr),v(cm -1 ): 3313(O–H), 2939, 2863(CH 2 ), 1601 (C=C), 1249 (Ar–O). 1 HNMR (600MHz, DMSO-d 6 )δ7.82(d,J=8.9Hz,4H),7.09(d,J=9.0Hz,4H),4.37(s,2H),4.05(t,J=...
Embodiment 2
[0077] Example 2: Synthesis of azobenzenediol AB2
[0078] Taking the 4,4'-dihydroxyazobenzene that the weight part is 5 parts, the 11-bromo-1-undecanol that the weight part is 15 parts, the potassium carbonate or the potassium hydroxide that the weight part is 20 parts, the weight part As 0.1 part of potassium iodide, the four raw materials were dissolved in 200 parts by weight of 2-butanone or N,N-dimethylacetamide, and the reaction was stirred at 80 °C for 12 hours, and the solution was precipitated in water. It was recrystallized twice from absolute ethanol. The obtained solid was vacuum-dried at 40 °C for 12 h to obtain high-purity azobenzenediol AB2. The reaction equation for monomer AB2 is:
[0079]
[0080] The infrared spectral data of monomer AB2 are as follows:
[0081] FT-IR(KBr),ν(cm -1 ): 3335 (O–H); 2918, 2850 (CH 2 ); 1602 (C=C); 1246 (Ar–O).
Embodiment 3
[0082] Example 3: Synthesis of azobenzenediol AB3
[0083] Take the 4,4'-dihydroxyazobenzene that is 5 parts by weight, the 2-chloroethoxyethanol of 15 parts by weight, the potassium carbonate or potassium hydroxide of 20 parts by weight, and the weight part is 0.1 parts of potassium iodide, the four raw materials were dissolved in 200 parts by weight of 2-butanone, N,N-dimethylformamide or N,N-dimethylacetamide, and the reaction was stirred at 80°C for 12h , the solution was precipitated in water and recrystallized twice with absolute ethanol. The obtained solid was vacuum-dried at 40 °C for 12 h to obtain high-purity azobenzenediol AB3. The reaction equation for monomer AB3 is:
[0084]
[0085] The H NMR data of monomer AB3 are as follows:
[0086] 1 H NMR (400MHz, DMSO-d 6 )δ7.83(d,J=8.9Hz,4H),7.12(d,J=9.0Hz,4H),4.66(s,2H),4.27–4.15(m,4H),3.84–3.73(m,4H ),3.52(q,J=2.9Hz,8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com