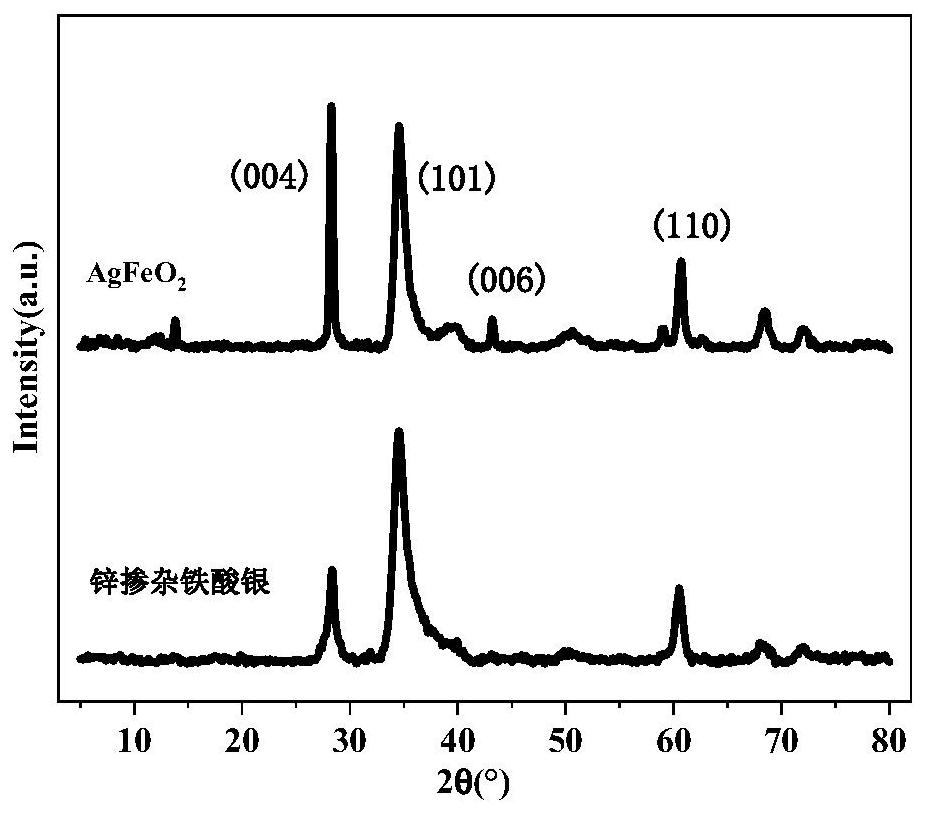
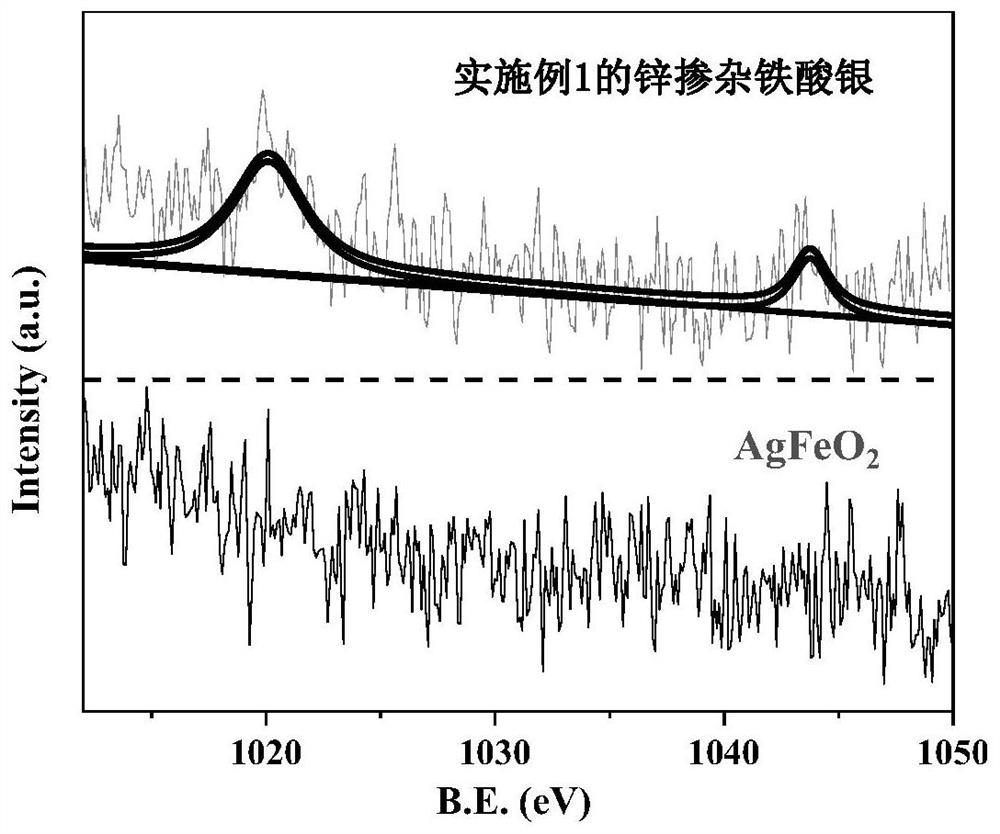
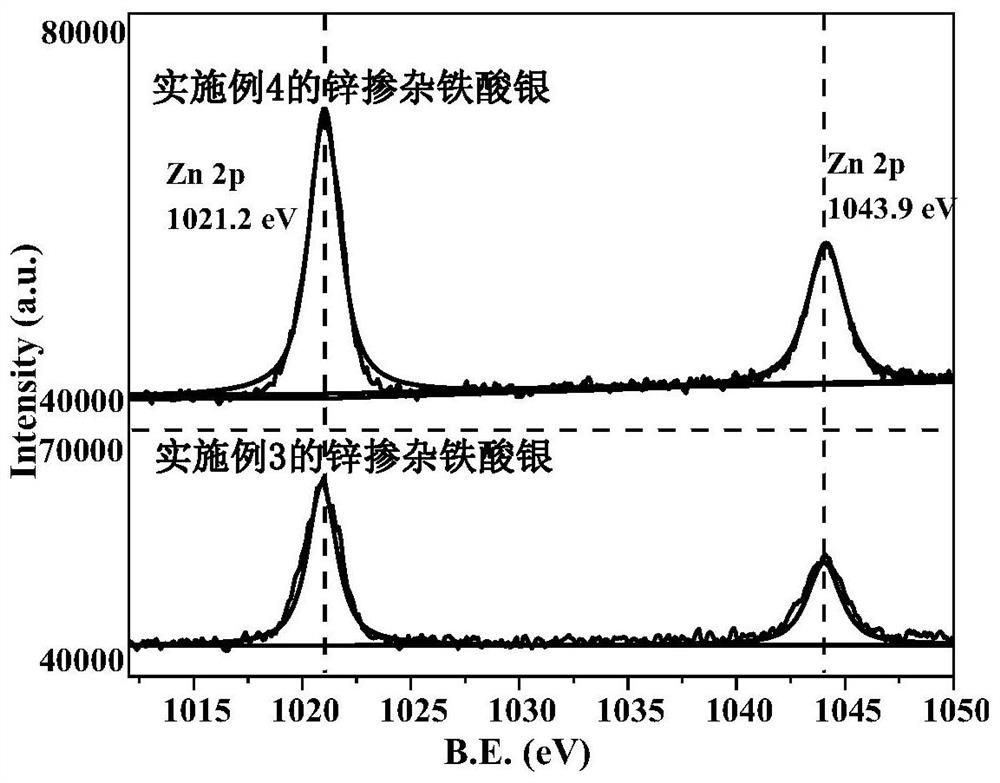
Zinc-doped silver ferrite catalyst, preparation method and application thereof
A technology of silver ferrite and catalyst, applied in the field of zinc-doped silver ferrite catalyst and its preparation, can solve the problems of difficult recovery, small specific surface area of silver monoferrite, difficult separation and the like, achieve resource saving and easy control of reaction process , the effect of strong catalytic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The invention provides a method for preparing a zinc-doped silver ferrite catalyst, comprising:
[0058] providing a mixture comprising silver salts, zinc salts and iron salts;
[0059] The mixed liquid is mixed and reacted with an alkaline substance, and then solid-liquid separation is carried out, and the solid is the zinc-doped silver ferrite catalyst.
[0060] The mixed solution is obtained by mixing silver salt solution, zinc salt solution and iron salt solution;
[0061] Preferably, the silver ion concentration in the silver salt solution is 0.06-0.10mol / L, the zinc ion concentration in the zinc salt solution is 0.01-0.02mol / L; when the iron ion concentration in the iron salt solution is 0.06-0.10mol / L, it can ensure that each salt The solution is mixed thoroughly.
[0062] Above-mentioned silver salt solution can comprise at least one in silver nitrate solution and silver sulfate solution; The content is 50-60% of the total mass of silver-containing nitrate), ...
Embodiment 1
[0082] (1) Accurately weigh 20 parts of silver-containing nitrate (wherein the content of silver ions is 60% of the total mass of silver-containing nitrate), and make it completely dissolved in 100 parts of deionized water by stirring to obtain a silver nitrate solution for subsequent use;
[0083] (2) Accurately weigh 10 parts of zinc nitrate tetrahydrate, and make it completely dissolved in 100 parts of deionized water by stirring to obtain ferric nitrate solution for subsequent use;
[0084] (3) Accurately weigh 20 parts of ferric nitrate nonahydrate, and make it completely dissolved in 100 parts of deionized water by stirring to obtain ferric nitrate solution for subsequent use;
[0085] (4) Accurately weigh 150 parts of sodium hydroxide, make it be dissolved in 100 parts of deionized water completely by stirring, obtain sodium hydroxide solution for subsequent use;
[0086] (5) According to the molar ratio of silver ions in silver nitrate solution, zinc ions in zinc nitra...
Embodiment 2
[0089] The difference of this embodiment with embodiment 1 is: step (5) is replaced by: press the mol ratio of hydroxide ion in silver ion in silver nitrate solution, zinc ion in zinc nitrate solution, iron ion in ferric nitrate solution and sodium hydroxide solution Take silver nitrate solution, zinc nitrate solution, ferric nitrate solution and sodium hydroxide solution at the ratio of 7.5:1:10:120; others are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com