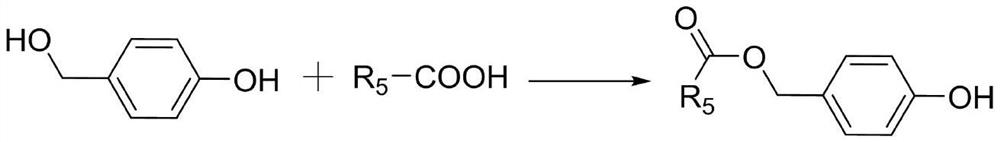
ROS response type heparin polysaccharide nano prodrug compound based on click reaction, nano preparation and preparation method and application of ROS response type heparin polysaccharide nano prodrug compound
A click reaction, polysaccharide-like technology, which can be used in drug combinations, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., can solve problems such as low synthesis conversion efficiency, inability to control the synthesis ratio of different drugs, and waste of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Preparation of ROS-responsive unfractionated heparin-lonidamine-androquinol prodrug complex based on click reaction
[0063] Weigh 2 mmol of unfractionated heparin and dissolve it in 10 mL of N,N-dimethylformamide, dissolve at 60°C, add N,N’-carbonyldiimidazole under nitrogen protection and ice bath conditions to activate the carboxyl group of unfractionated heparin. After activation in ice bath for 1 h, add 5 mL of 2-azidoethylamine solution dissolved in N,N-dimethylformamide (unfractionated heparin: N,N'-carbonyldiimidazole: 2-azidoethylamine The molar ratio is 1:8:5), and slowly added dropwise to the unfractionated heparin solution. After reacting for 48 hours, 5 times the volume of glacial ether was added to precipitate, and the precipitate was obtained by centrifugation. Lower the probe and sonicate for 20 minutes, dialyze in distilled water, and dry in vacuum to obtain azide-modified unfractionated heparin. Weigh an appropriate amount of azide-modified...
Embodiment 2
[0070] Example 2: Preparation of ROS-responsive low molecular weight heparin-aspirin-gambogic acid prodrug complex based on click reaction
[0071] Weigh 2 mmol of low molecular weight heparin and dissolve it in 10 mL of formamide, dissolve in an oil bath at 60°C, and add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide activates the carboxyl group of low molecular weight heparin. After activation at 0°C for 0.5 h, add 5 mL of 2-azidoethanol solution dissolved in formamide (low molecular weight heparin: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: The molar ratio of N-hydroxysuccinimide:2-azidoethanol is 1:4:4:2), slowly added dropwise to the low molecular weight heparin solution, and after 24 hours of reaction, 5 times the volume of ice methanol was added to precipitate , the precipitate was obtained by suction filtration, redissolved with an appropriate amount of distilled water, centrifuged at 3000rpm for 10min, ultrasoni...
Embodiment 3
[0080] Example 3: Preparation of ROS-responsive desulfated heparin-DHEA-selumetinib prodrug complex based on click reaction
[0081]Weigh 2 mmol of desulfurized heparin and dissolve it in 10 mL of N,N-dimethylacetamide, dissolve in an oil bath at 60°C, add N,N'-dicyclohexyl carboximide and 4-dimethyl Aminopyridine, activates the carboxyl group of desulfated heparin. After activation in ice bath for 2 h, add 2-azido-N-(2-hydroxyethyl)ethyl dissolved in a mixed solvent of formamide and N,N-dimethylacetamide (v:v=1:1) Amide solution 5mL (desulfated heparin: N,N'-dicyclohexyl carboximide: 4-dimethylaminopyridine: 2-azido-N-(2-hydroxyethyl) acetamide molar ratio is 1:10:10:3), slowly added dropwise to the desulfated heparin solution, and reacted in the dark for 36 hours, then added 5 times the volume of ice methanol to precipitate, and the precipitate was obtained by suction filtration, redissolved with an appropriate amount of distilled water, centrifuged at 3000rpm for 10min, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com