Method for selectively reducing alpha, beta-unsaturated ketone
An unsaturated and selective technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of long reaction time and low reaction conversion rate, and achieve easy operation, simplified experimental operation process, and production high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
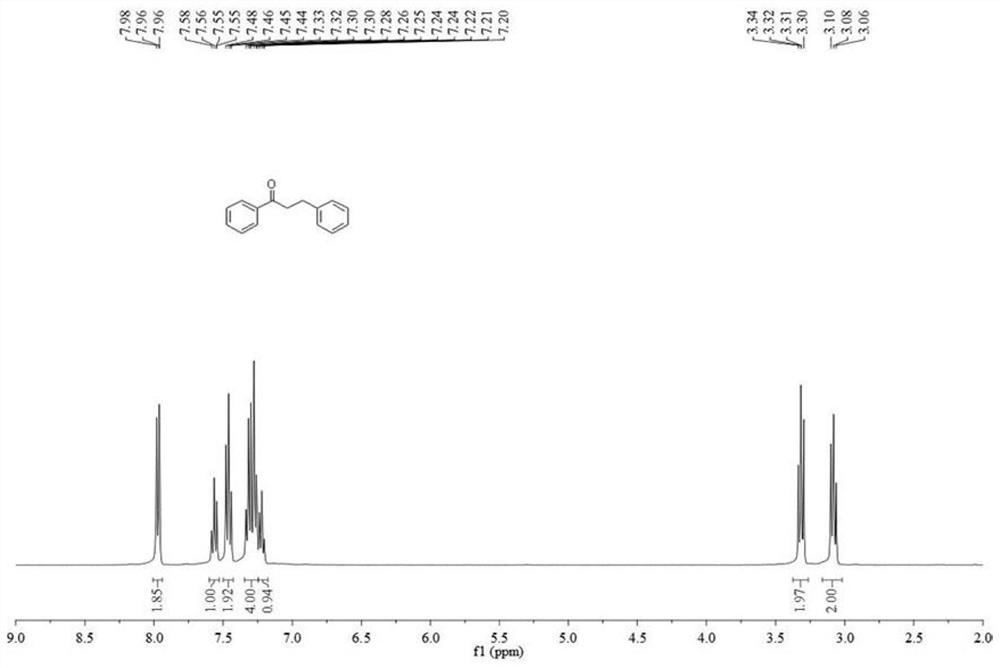
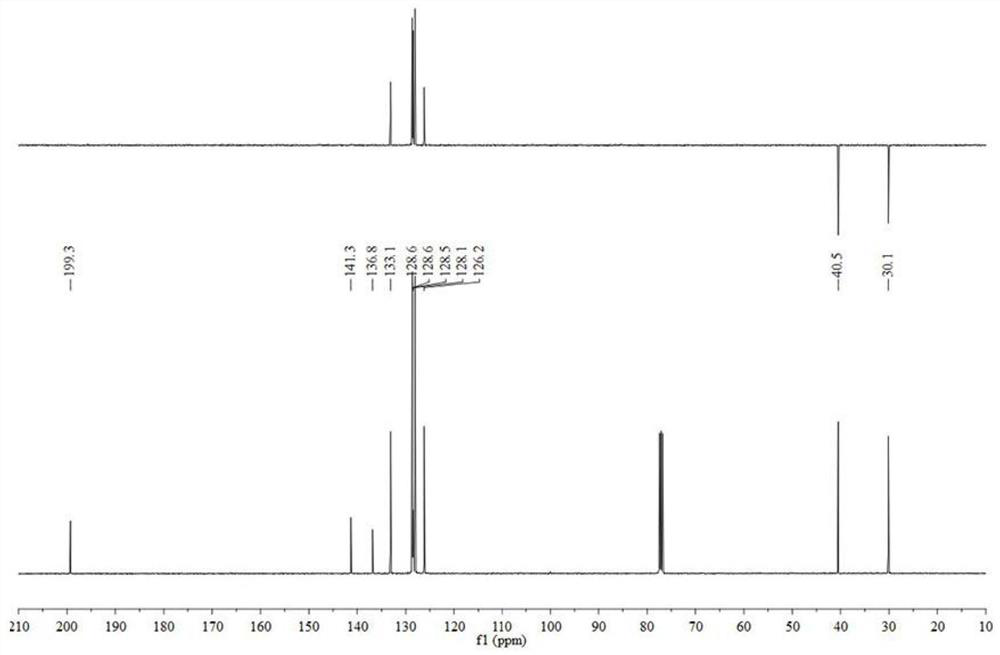
[0035] 1 mmol chalcone was added to a 10 mL reaction test tube, a protic solvent was added to dissolve it completely, then 5% mol of Lews acid, 0.02 mmol of TMSCl and 1.0 mmol of triethylsilane were sequentially added, and the reaction was stirred at room temperature. When the reaction is complete, it is quenched and extracted with saturated sodium bicarbonate solution, and the organic layer is collected. Then, the solvent is removed by distillation under reduced pressure, and then dihydrochalcone can be obtained by recrystallization (petroleum ether:ethyl acetate=20:1 mixed solvent).
[0036] Characterization data
[0037] Dihydrochalcone: 1 H NMR (400 MHz, CDCl 3 ) δ 7.97 (m, 2H), 7.56 (m, 1H), 7.46 (m, 1H), 7.29 (m, 2H), 7.22 (m, 4H), 3.32 (m, 2H), 3.08 (m, 2H) ; 13 C NMR (101MHz, CDCl 3 ) δ 199.3, 141.3, 136.8, 133.1, 128.6, 128.1, 126.2, 40.5, 30.1.
Embodiment 2
[0039] Put 1mmol of 4'-hydroxy-2,4-dimethoxychalcone in a 10mL reaction test tube, add a protic solvent to dissolve it completely, then add 5% mol Lews acid, 0.02mmol TMSCl and 1.0mmol triethyl in turn silane, and the reaction was stirred at room temperature. When the reaction is complete, it is quenched with saturated sodium bicarbonate solution, and the organic layer is extracted and collected. Then, the solvent is removed by distillation under reduced pressure, and then recrystallized (petroleum ether: ethyl acetate = 3:1 mixed solvent) to obtain 4'-hydroxy-2,4-dimethoxydihydrochalcone (dragon's blood). prime A).
[0040] Characterization data
[0041] 4'-Hydroxy-2,4-dimethoxydihydrochalcone: 1 H NMR (400 MHz, CDCl 3 ) δ 7.92 (m, 2H), 7.08 (d, J = 8.1 Hz, 1H), 6.88 (m, 2H), 6.42 (m, 2H), 3.78 (d, J = 7.0 Hz, 6H), 3.17 (m , 2H), 2.96 (m, 2H); 13 C NMR (101 MHz, CDCl 3 ) δ 194.4, 155.3, 154.2, 153.1, 125.5, 125.1, 116.7, 110.1, 98.7, 93.4, 50.2, 50.00, 33.7, 20.2.
Embodiment 3
[0043] Put 1mmol of 1-(naphthalen-2-yl)-3-phenylpropen-2-en-1-one in a 10mL reaction test tube, add a protic solvent to make it completely dissolved, and then add 5% mol Lews acid, 0.02 mmol TMSCl and 1.0 mmol triethylsilane, and the reaction was stirred at room temperature. When the reaction is complete, it is quenched with saturated sodium bicarbonate solution, and the organic layer is extracted and collected. Then, the solvent is removed by distillation under reduced pressure, and the compound 1-(naphthalen-2-yl)-3-phenylpropan-1-one can be obtained by recrystallization (petroleum ether:ethyl acetate=15:1 mixed solvent).
[0044] Characterization data
[0045] 1-(Naphthalen-2-yl)-3-phenylpropan-1-one: 1 H NMR (400 MHz, CDCl 3 ) 8.38 (s, 1H), 7.96 (m, 1H), 7.87 – 7.77 (m, 3H), 7.49 (m, 2H), 7.23 (t, J = 4.3 Hz, 4H), 7.16 (d, J= 11.5 Hz, 1H), 3.36 (m, 2H), 3.11 – 2.97 (m, 2H); 13 C NMR (101 MHz, CDCl 3) δ198.2, 140.3, 134.5, 133.1, 131.5, 128.7, 128.5, 127.5, 127.4, 126...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com