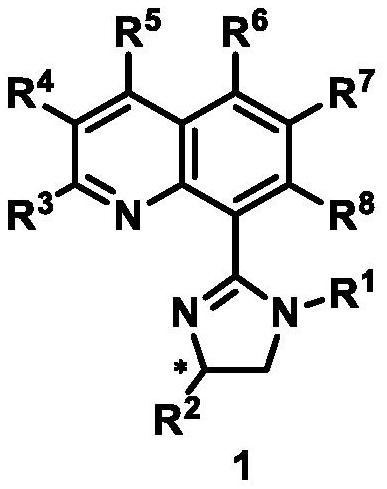
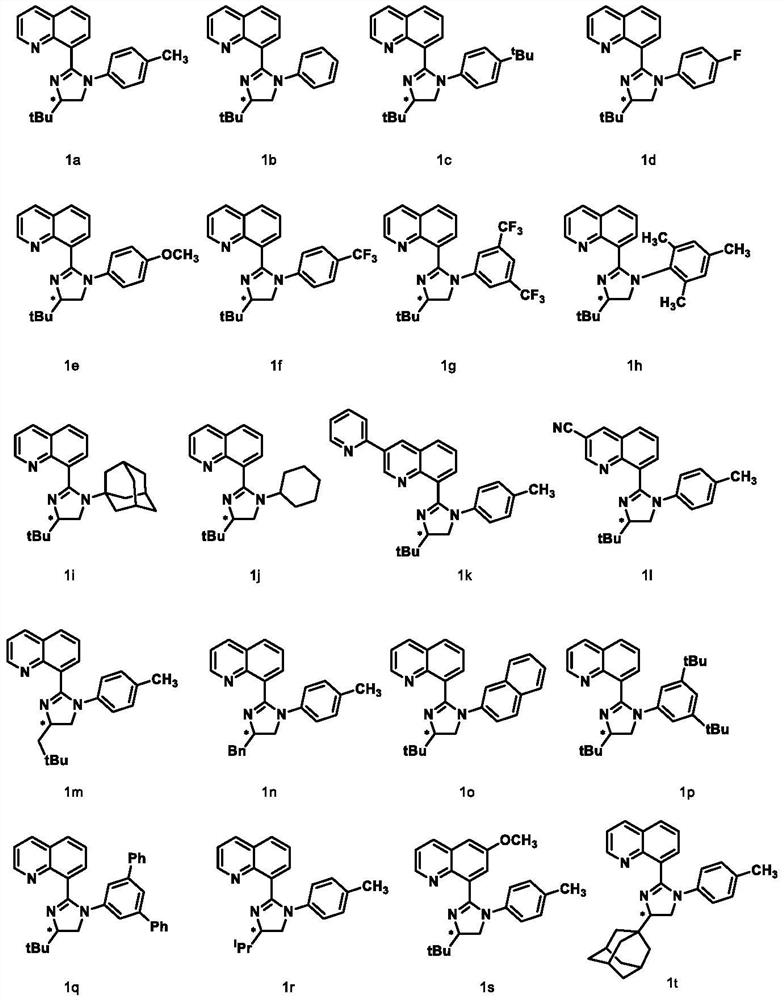
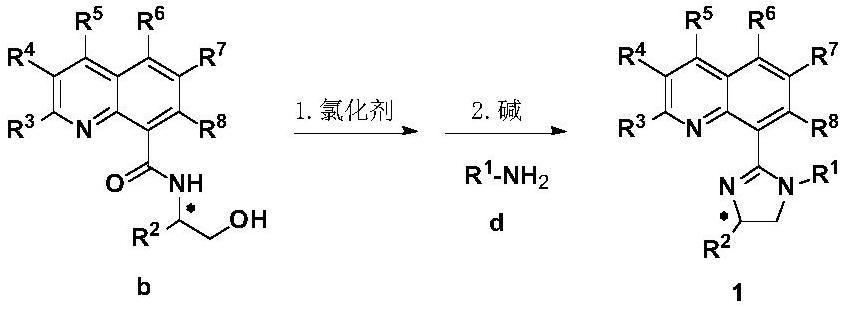
Synthesis method and application of chiral quinoline-imidazoline ligand
A technology of imidazoline and quinoline, applied in the field of organic synthesis, can solve problems such as difficult control of stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: Synthesis of (S)-N-(1-hydroxyl-3,3-dimethyl-but-2-yl)quinoline-8-carboxamide:
[0086]
[0087] Quinoline-8-carboxylic acid (173.1mg, 1mmol) was added to a dry reaction flask of 100mL, replaced by a nitrogen atmosphere, dichloromethane (20mL), N-methylmorpholine (0.30mL, 2.7mmol) were added, and Place the reaction bottle in an ice-water bath, add isobutyl chloroformate (0.15mL, 1.15mmol), stir in an ice-water bath for 1 hour, then add (S)-2-amino-3,3-dimethyl-1-butanol Alcohol (175.8mg, 1.5mmol), returned to room temperature, stirred for 24 hours, and the reaction was complete as monitored by TLC. Add water (20mL) to the reaction solution to quench, separate the layers, extract the aqueous phase with dichloromethane (20mL×3), combine the organic phases, then wash with saturated sodium chloride solution, separate the layers, and dry the organic phase with anhydrous sodium sulfate , concentrated under reduced pressure, column chromatography (petroleum et...
Embodiment 2
[0089] Example 2: Synthesis of (S)-8-(4-tert-butyl-1-p-tolyl-4,5-dihydro-1H-imidazol2-yl)quinoline:
[0090]
[0091] In a 50mL reaction tube, (S)-N-(1-hydroxy-3,3-dimethyl-but-2-yl)quinoline-8-carboxamide (S)-1a-b (1.5mmol) Dissolve in thionyl chloride (1.5mL), reflux at 90°C for 12 hours, until the TLC reaction is complete, desolvate under reduced pressure, the solid does not need further post-treatment, directly add to a 50mL reaction tube, add ether (10mL) to dissolve, add Triethylamine (15mmol) was added to p-methylaniline (1.65mmol), and stirred at room temperature for 12 hours until the TLC reaction was complete. Add 10% sodium hydroxide solution to the reaction solution to quench, extract with dichloromethane (30mL×3), combine the organic phases, then wash with saturated sodium chloride solution, separate the layers, dry the organic phase with anhydrous sodium sulfate, reduce Concentrate under reduced pressure, and column chromatography (dichloromethane / methanol=10...
Embodiment 3
[0092] Example 3: Synthesis of (S)-8-(4-tert-butyl-1-phenyl-4,5-dihydro-1H-imidazol2-yl)quinoline:
[0093]
[0094] The preparation method is the same as in Example 2, starting material (S)-1a-b 0.2mmol, light yellow solid, 43.8mg, yield 66%, 1 H NMR (400MHz, CDCl 3 ): δ8.74(dd, J=4.0, 1.6Hz, 1H), 8.07(dd, J=8.4, 1.6Hz, 1H), 7.95(d, J=7.2Hz, 1H), 7.87(d, J= 8.4,1H),7.56(t,J=7.6Hz,1H),7.29(dd,J=8.0,4.0Hz,1H),6.92(t,J=8.0Hz,2H),6.77(d,J=7.2 Hz, 1H), 6.67(d, J=8.0Hz, 1H), 4.31-4.19(m, 2H), 3.96(dd, J=8.4, 7.6Hz, 1H), 1.11(s, 9H); 13 C NMR (100MHz, CDCl 3 ):δ161.2,150.8,145.9,141.0,136.0,131.3,130.4,128.5,128.2,126.3,123.1,121.5,120.5,72.3,53.2,34.6,26.1; HRMS(ESI):[M+H] + Calcd for C 22 h 24 N 3 + :330.1965; found: 330.1967.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com