Intrinsically Microporous Polyaluminum/Borate Solid-State Electrolytes and Batteries
A technology of solid electrolytes and inherent micropores, applied in solid electrolytes, composite electrolytes, non-aqueous electrolytes, etc., can solve problems such as low ionic conductivity and insufficient negative charge delocalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
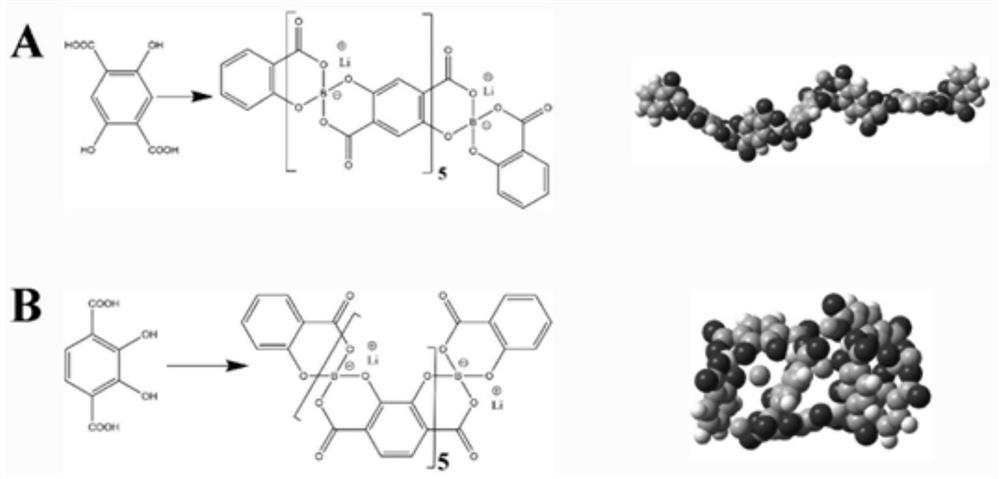
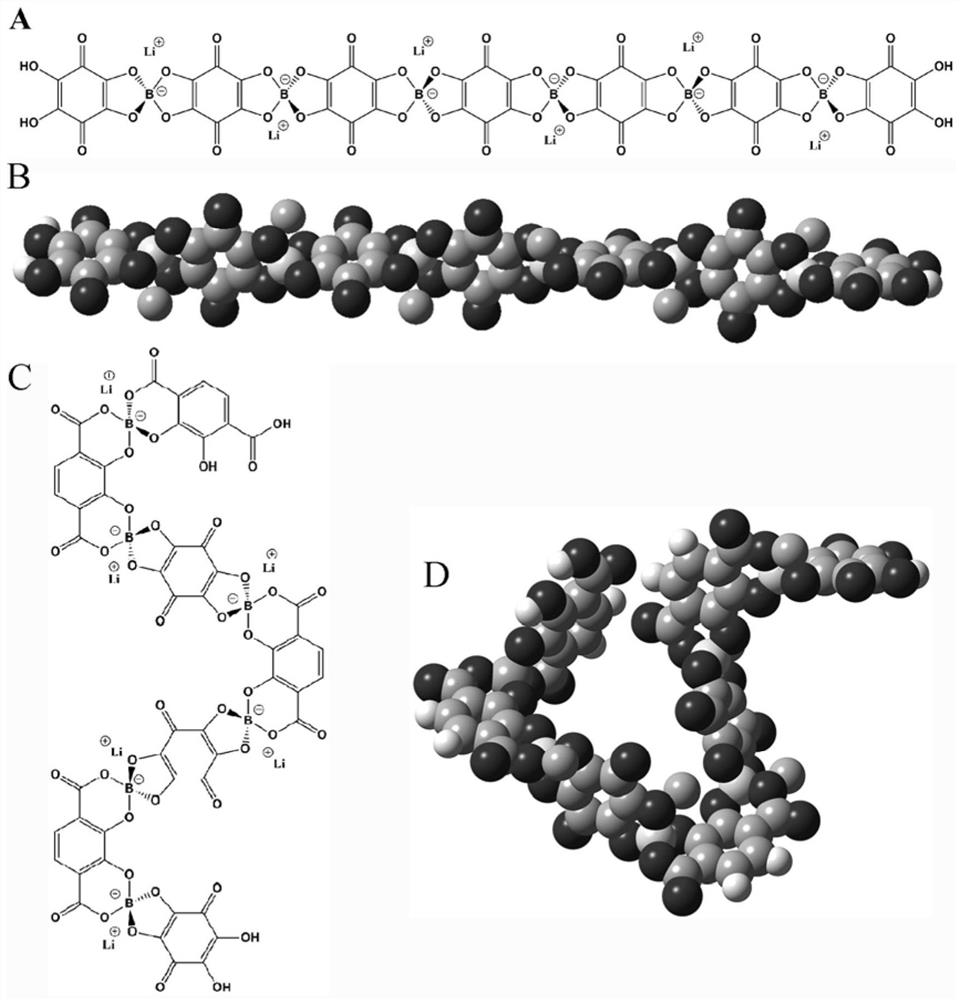
[0030] This embodiment provides a poly 2,3-dihydroxy-1,4-phthalic acid lithium borate solid electrolyte ( M is a boron atom), synthesized according to the following steps:
[0031] Add 19.80 g of 2,3-dihydroxy-1,4-phthalic acid to 200 g of dimethylsulfoxide 6.183 g of boric acid and 4.196 g of lithium hydroxide monohydrate were stirred and reacted at room temperature for 6 hours to obtain a light yellow solution, which was rotary evaporated to obtain poly-2,3-dihydroxy-1,4-phthalic acid lithium borate.
[0032] Structural characterization: 13C NMR spectra have resonance peaks at chemical shifts of 113.7, 118.0, 143.6 and 156.6ppm, corresponding to the carbon on the benzene ring connected to the carboxyl group, the carbon on the benzene ring connected to hydrogen, and the carbon directly connected to the hydroxyl group on the benzene ring. chain carbon and carboxyl carbon. The analysis result of carbon, hydrogen and nitrogen elements is C: 45.15%, which is the same as the m...
Embodiment 2
[0038] This embodiment provides a poly 2,3-dihydroxy-1,4-phthalic acid lithium aluminate solid electrolyte ( M is an aluminum atom), synthesized according to the following steps:
[0039] Add 19.80 g of 2,3-dihydroxy-1,4-phthalic acid to 200 g of dimethylsulfoxide 7.800 g of aluminum hydroxide and 4.196 g of lithium hydroxide monohydrate were stirred and reacted at 100° C. for 6 hours to obtain a light yellow solution, which was evaporated by rotary evaporation to obtain polyaluminum 2,3-dihydroxy-1,4-phthalate.
[0040] Structural characterization: 13C NMR spectrum has resonance peaks at chemical shifts of 118.4, 143.9 and 175.8ppm, corresponding to the benzene ring carbon, the benzene ring carbon and the carboxyl carbon that are linear with the hydroxyl group, respectively. The analysis result of carbon, hydrogen and nitrogen elements is C: 42.05%, which is the same as the molecular formula of the polymer (C 8 o 6 The theoretical carbon content (42.11%) corresponding to...
Embodiment 3
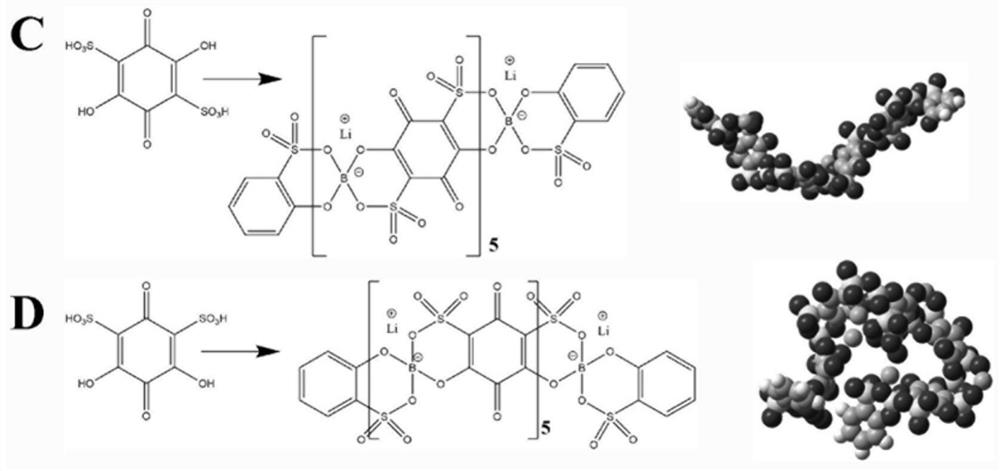
[0043] This embodiment provides a poly 3,6-dihydroxy-1,2-phthalic acid lithium borate solid electrolyte ( M is a boron atom), synthesized according to the following steps:
[0044] Add 19.80 g of 3,6-dihydroxy-1,2-phthalic acid to 200 g of dimethylsulfoxide 6.183 g of boric acid and 4.196 g of lithium hydroxide monohydrate were stirred and reacted at room temperature for 6 hours to obtain a light yellow solution, which was rotary evaporated to obtain poly-3,6-dihydroxy-1,2-phthalic acid lithium borate.
[0045] Structural characterization: 13C NMR spectra have resonance peaks at chemical shifts of 108.2, 124.4, 149.9 and 162.7ppm, corresponding to the carbon connected to the carboxyl group on the benzene ring, the carbon connected to hydrogen on the benzene ring, and the hydroxyl group on the benzene ring Straight chain carbons and carboxyl carbons. The analysis result of carbon, hydrogen and nitrogen elements is C: 45.15%, which is the same as the molecular formula of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com