Benzo-beta-lactam and preparation method thereof
A lactam and benzo technology, applied in the chemical field, can solve the problems of low yield, many side reactions, and many by-products, and achieve the effects of high yield, easy operation, and short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation reaction formula of N-hydrobenzo β-lactam is as follows:
[0043]
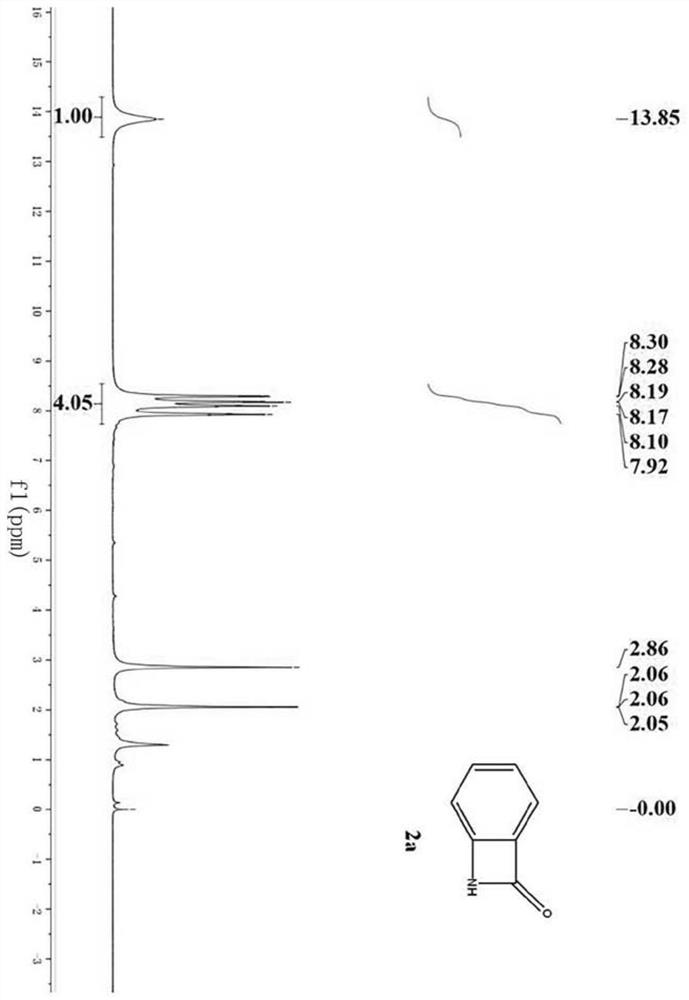
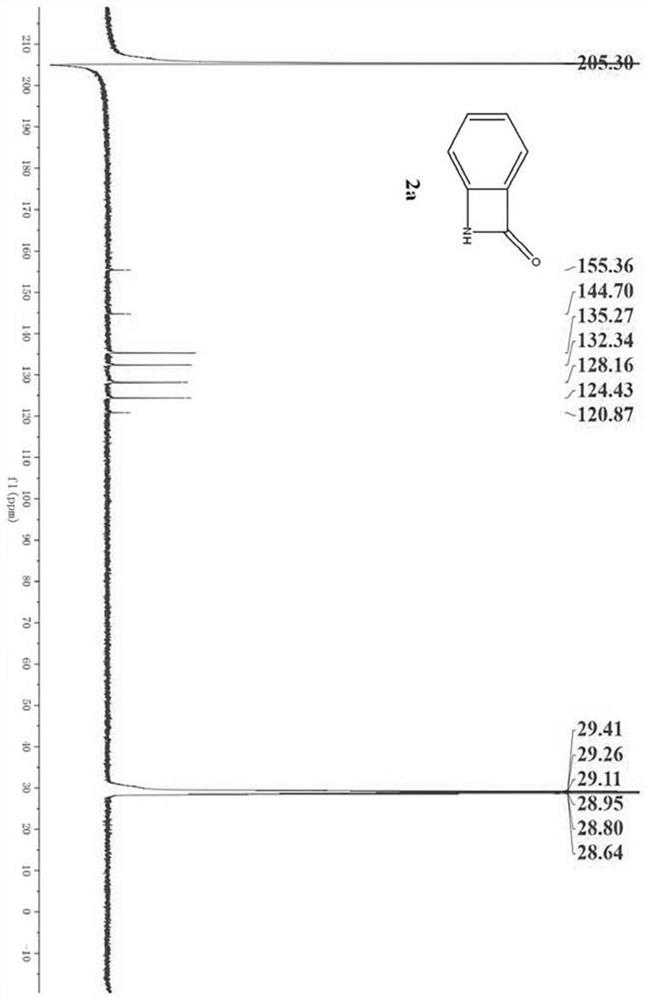
[0044] Dissolve 27.2mg (0.2mmol) of anthranilamide (1a) in 2.0mL of acetonitrile (analytical grade), and stir in a 10mL reaction kettle at room temperature (20°C-25°C). Then 23uL (0.4mmol, 2equiv) glacial acetic acid was added, and 36uL (0.3mmol, 1.5equiv) tert-butyl nitrite was added two minutes later. Stirring was continued for 0.5 h and the reaction was complete. After the reaction was completed, 10.0 mL of water and 10.0 mL of ethyl acetate were added for extraction, and the organic phase was washed with saturated sodium carbonate solution and saturated brine respectively, then dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a crude product, which was finally washed with Ethyl acetate:petroleum ether=1:2 washing solution was purified by column chromatography to obtain the pure product N-hydrobenzoβ-lactam (2a). Th...
Embodiment 2
[0046] The preparation reaction formula of N-hydrobenzo β-lactam is as follows:
[0047]
[0048]1.39 g (10.0 mmol) of anthranilamide (1a) was dissolved in 20.0 mL of acetonitrile (analytical grade), and stirred in a 100 mL reaction kettle at room temperature (20°C-25°C). Then 1.15mL (20.0mmol, 2equiv) of glacial acetic acid was added, and 1.80mL (15.0mmol, 1.5equiv) of tert-butyl nitrite was added two minutes later. Stirring was continued for 1.5 h and the reaction was complete. After the reaction was completed, 100.0 mL of water and 100.0 mL of ethyl acetate were added for extraction. The organic phase was washed with saturated sodium carbonate solution and saturated brine respectively, then dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a crude product. Ethyl acetate:petroleum ether=1:2 washing solution was purified by column chromatography to obtain the pure product N-hydrobenzoβ-lactam (2a). This comp...
Embodiment 3
[0050] The preparation reaction formula of N-hydrobenzo β-lactam is as follows:
[0051]
[0052] 27.2 mg (0.2 mmol) of anthranilamide (1a) was dissolved in 2.0 mL of distilled water, and stirred in a 10 mL reaction kettle at room temperature (20°C-25°C). Then 23uL (0.4mmol, 2equiv) of glacial acetic acid was added, and 20.7mg (0.3mmol, 1.5equiv) of sodium nitrite was added two minutes later. Stirring was continued for 0.5 h and the reaction was complete. After the reaction, add 10.0mL water and 10.0mL ethyl acetate for extraction, wash the organic phase with saturated sodium carbonate solution and saturated brine respectively, then dry with anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain the crude product. Ethyl acetate:petroleum ether=1:2 washing solution was purified by column chromatography to obtain the pure product benzo β-lactam (2a). The compound is a known compound, and the yield is 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com