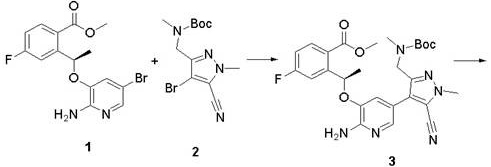
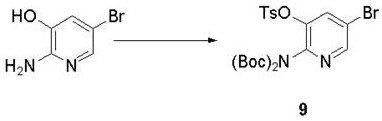
Synthesis method of Lorlatinib intermediate 2-amino-5-bromo-3-hydroxypyridine
A technology for the synthesis of hydroxypyridine, which is applied in the field of 2-amino-5-bromo-3-hydroxypyridine synthesis, can solve problems such as drug resistance, and achieve the effects of increasing the rate, improving product quality, and increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
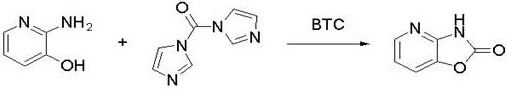
[0035] Example 1: Synthesis of 3H-oxazol[4,5-b]pyridin-2-one
[0036] 2-Amino-3-hydroxypyridine (4.0g, 36.3mmol) was dissolved in THF (120 mL), and after it was completely dissolved, CDI (8.8g, 54.3mmol) was added after stirring for a while, and the CDI solid gradually dissolved, and the solution was Yellowish-brown clear shape, stirred at room temperature for 1.5 hours, added BTC (6.5g, 21.9mmol) in batches under ice bath conditions, after the addition was completed, heated to reflux, reacted for 1 hour, after the reaction was completed, filtered, washed the filter cake with water, and dried Dry to obtain 4.75 g of light yellow powder 3H-oxazol[4,5-b]pyridin-2-one with a yield of 96.1%. The crude product can be directly used in the next bromination reaction without purification. Intermediate structure identification requires further separation and purification by column chromatography, 1H NMR (400 MHz, DMSO) δ 12.43 (s, 1H), 8.04 (dd, J =5.3, 1.2 Hz, 1H), 7.64 (dd, J = 7.9, ...
Embodiment 2
[0037] Example 2: Synthesis of 6-bromo-3H-oxazolo[4,5-b]pyridin-2-one
[0038] The crude oxazol[4,5-b]pyridin-2(3H)-one (2.50 g, 18.35 mmol) obtained in the aforementioned ring-closing step, 30 mL of anhydrous N,N-dimethylformamide (DMF), Add in the quartz glass three-necked bottle successively, then add 25 mg photoinitiator 2-hydroxyl-2-methyl-1-phenyl-1-propanone, adopt the 23 watts ultraviolet lamp that has standard lampshade to irradiate reaction bottle, control The temperature of the reaction solution is at 0-5°C, and the DMF solution containing liquid bromine (2.94g, 18.35 mmol) is slowly added dropwise. After the reaction is complete, pour the reaction solution into a certain amount of ice-water mixture, stir, filter, wash the filter cake with water, and dry to obtain a yellow solid 6-bromo-3H-oxazolo[4,5-b]pyridine- The crude product of 2-ketone was 3.93g, the molar yield of the crude product in the photocatalytic bromination single-step reaction was 86.4%, and the re...
Embodiment 3
[0039] Example 3: Synthesis of 2-amino-5-bromo-3-hydroxypyridine
[0040] The 6-bromo-3H-oxazolo[4,5-b]pyridin-2-one intermediate (5.0g, 23.3mmol) prepared according to the photocatalytic bromination reaction method was added to the three-necked flask, Then add 40 mL of aqueous sodium hydroxide solution with a concentration of 10% by mass, and heat up to reflux for 14 hours. Monitor the reaction process by thin-layer chromatography. After the reaction raw material 6-bromo-3H-oxazolo[4,5-b]pyridin-2-one is completely converted, cool to room temperature and add 5% dilute hydrochloric acid dropwise to adjust the pH value of the solution. to 6-7, extracted three times with ethyl acetate, collected the organic layer, dried the organic layer by adding anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 3.96 g of the crude product of 2-amino-5-bromo-3-hydroxypyridine as gray-brown solid, The single-step reaction of hydrolysis has a molar yield of 90%, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com