a fe 2+ /fe 3+ Electrochemical preparation device, preparation method and application of hydroxide
A hydroxide and preparation device technology, which is applied in the field of environmental remediation, can solve the problems of high preparation cost, low product recovery rate, and practical operation difficulties, and achieve the effects of fast preparation speed, favorable product production, and strong reducing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
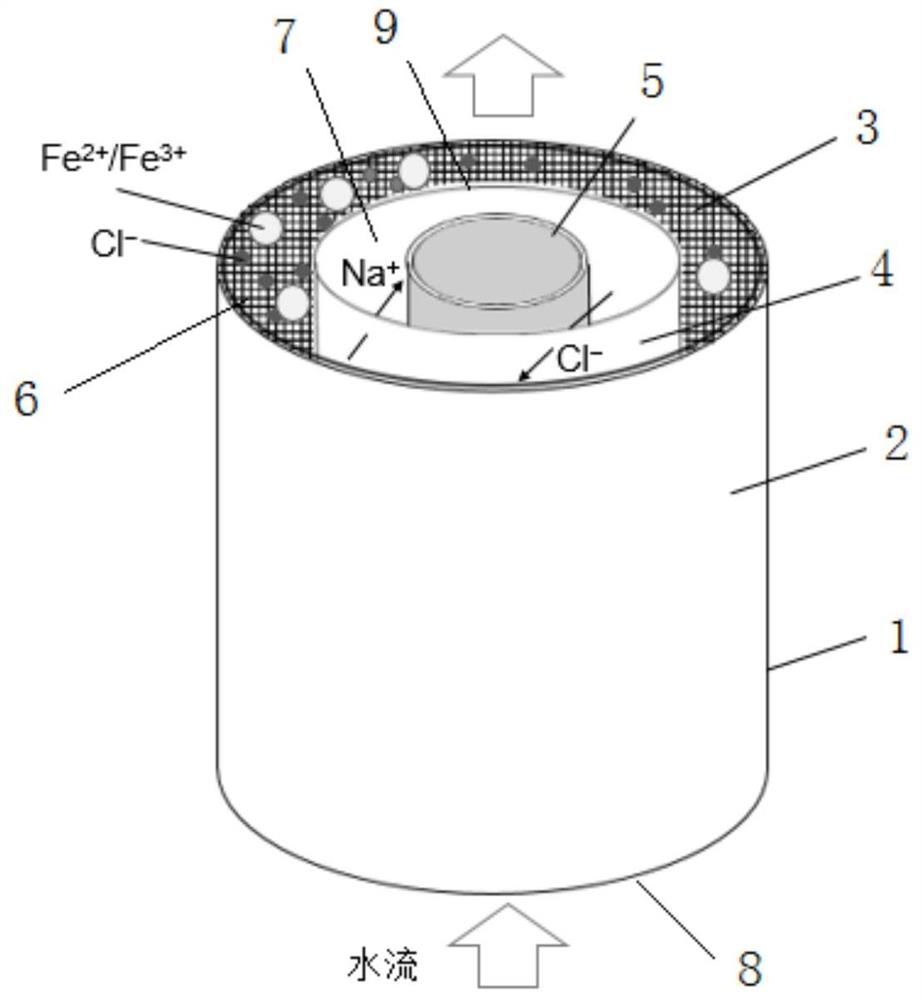
[0034] Such as figure 1 As shown, a Fe 2+ / Fe 3+ Electrochemical preparation device for hydroxide, used to prepare Fe 2+ / Fe 3+ Hydroxide, comprising a reactor 1, the reactor 1 is provided with an iron-containing anode 2, a porous electrode 3, a cation exchange membrane 4 and a cathode 5 in sequence from the outside to the inside, and the porous electrode 3 is attached to the iron-containing anode 2 Inside, the cation exchange membrane 4 divides the reaction chamber into an anode chamber 6 and a cathode chamber 7, and the reactor 1 is provided with a water inlet 8 and a water outlet 9, and the water inlet 8 is located at the bottom of the reactor, connected to the anode The chamber communicates, and the water outlet 9 is located at the top of the reactor and communicates with the cathode chamber 7. The iron-containing anode 2 is any one of stainless steel or pure iron or cast iron or cast steel or Masteel or gray cast iron or gray iron material; the cathode 5 is an inert e...
Embodiment 2
[0037] The anode 2 is made of stainless steel, the porous electrode 3 is made of carbon felt, and the cathode 5 is made of graphite;
[0038] (1) On the anode 2 and the cathode 5 of the reactor 1, feed a direct voltage of 16V;
[0039] (2) The NaCl solution of 1mol / L is passed into the anode chamber 6 at the water inlet 8 at a flow rate of 4mL / min, and positively charged cations Fe 2+ , Fe 3+ and Na + Enter the cathode chamber 7 through the cation exchange membrane, and the anion OH in the cathode chamber 7 — Reaction, the effluent after the reaction flows out from the water outlet 9;
[0040] (3) The water outlet 9 is connected to the aeration bottle, and argon gas is introduced into the water outlet, argon gas is used for continuous aeration, and a magnetic stirrer is used for continuous stirring until fully deoxygenated;
[0041] (4) Leave the effluent after deoxygenation to stand, pour out the supernatant after the sediment precipitates, take the sediment and wash it w...
Embodiment 3
[0043] The anode 2 is made of stainless steel, the porous electrode 3 is made of glassy carbon, and the cathode 5 is made of graphite or stainless steel;
[0044] (1) On the anode 2 and the cathode 5 of the reactor 1, feed a direct voltage of 16V;
[0045] (2) The NaCl solution of 1mol / L is passed into the anode chamber 6 at the water inlet 8 at a flow rate of 4mL / min, and positively charged cations Fe 2+ , Fe 3+ and Na + Enter the cathode chamber 7 through the cation exchange membrane, and the anion OH in the cathode chamber 7 — Reaction, the effluent after the reaction flows out from the water outlet 9;
[0046] (3) The water outlet 9 is connected to the aeration bottle, and argon gas is introduced into the water outlet, argon gas is used for continuous aeration, and a magnetic stirrer is used for continuous stirring until fully deoxygenated;
[0047] (4) Leave the effluent after deoxygenation to stand, pour out the supernatant after the sediment precipitates, take the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com