Perovskite composite luminescent material as well as preparation method, product and application thereof
A luminescent material and perovskite technology, applied in luminescent materials, chemical instruments and methods, semiconductor devices, etc., can solve problems such as damage to the health of synthesizers, high fluorescence intensity and color gamut, narrow emission peak width, etc., to save Raw materials, simple operation, and the effect of narrow half-peak width
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1CsPbBr 3 / SrBr 2 Preparation of composite luminescent materials
[0059] (1) 63.9mg (0.3mmol) CsBr, 110.1mg (0.3mmol) PbBr 2 and 2.133g (6mmol) SrBr 2 ·6H 2 O raw materials are loaded into a planetary ball mill, fully ball milled to mix the raw materials evenly;
[0060] (2) Spread the homogeneous mixture obtained in step (1) in an alumina crucible, place it in a tube furnace and calcinate at 800° C. for 10 minutes under a nitrogen atmosphere to form a uniform molten liquid;
[0061] (3) After the product of step (2) is cooled to room temperature, it is placed in a planetary ball mill for grinding, so that the particle size of the obtained material is less than 80 μm, which is CsPbBr 3 / SrBr 2 Composite luminescent materials.
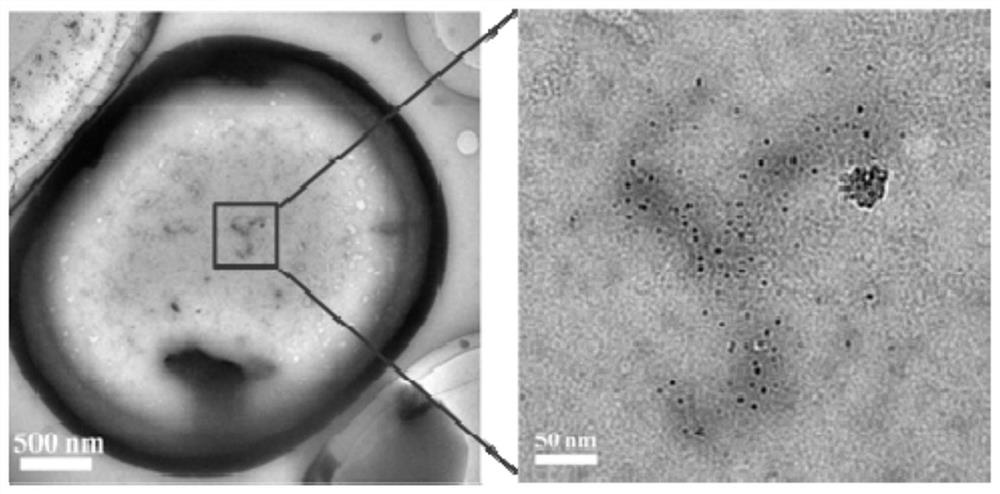
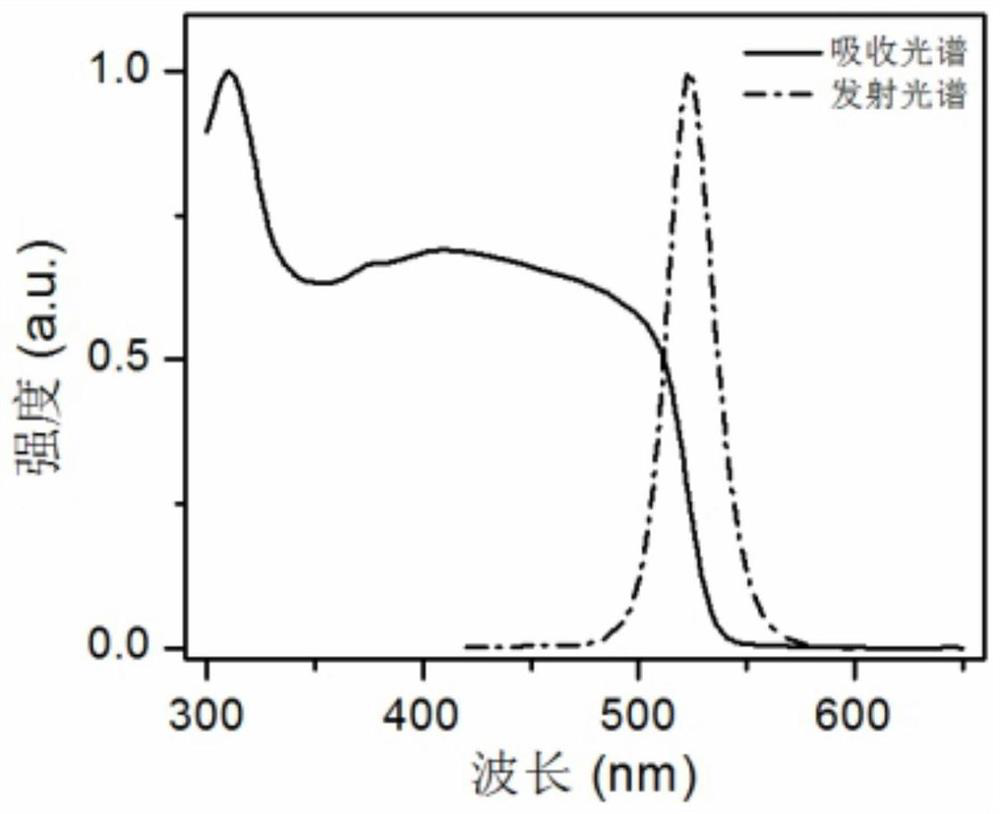
[0062] To the CsPbBr that embodiment 1 makes 3 / SrBr 2 Composite luminescent materials were tested for TEM and optical properties.
[0063] figure 1 For the preparation of CsPbBr 3 / SrBr 2 TEM image of the composite luminesc...
Embodiment 2
[0066] Example 2CsPbBr 3 / SrBr 2 Preparation of composite luminescent materials
[0067] (1) 63.9mg (0.3mmol) CsBr, 110.1mg (0.3mmol) PbBr 2 and 2.133g (6mmol) SrBr 2 ·6H 2 O raw materials are loaded into a planetary ball mill, fully ball milled to mix the raw materials evenly;
[0068] (2) Spread the homogeneous mixture obtained in step (1) in an alumina crucible, place it in a tube furnace and calcinate at 650° C. for 60 minutes under a nitrogen atmosphere to form a uniform molten liquid;
[0069] (3) After the product of step (2) is cooled to room temperature, it is placed in a planetary ball mill for grinding, so that the particle size of the obtained material is less than 80 μm, which is CsPbBr 3 / SrBr 2 Composite luminescent materials.
Embodiment 3
[0070] Example 3CsPbBr 3 / SrBr 2 Preparation of composite luminescent materials
[0071] (1) 425.6mg (2mmol) CsBr, 734mg (2mmol) PbBr 2 and 711 mg (2 mmol) SrBr 2 ·6H 2 O raw materials are loaded into a planetary ball mill, fully ball milled to mix the raw materials evenly;
[0072] (2) Spread the homogeneous mixture obtained in step (1) in an alumina crucible, place it in a tube furnace and calcinate at 950° C. for 10 minutes under a nitrogen atmosphere to form a uniform molten liquid;
[0073] (3) After the product of step (2) is cooled to room temperature, it is placed in a planetary ball mill for grinding, so that the particle size of the obtained material is less than 80 μm, which is CsPbBr 3 / SrBr 2 Composite luminescent materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com