Bone hemostatic material with osteogenesis inducing activity and preparation method thereof
A technology of hemostatic material and osteogenic activity, which is applied in the field of bone hemostatic material with inductive osteogenic activity and its preparation, and can solve the problems of bone implant material requirements, failure to achieve hemostatic effect, and unsatisfactory biocompatibility, etc. Achieve good biocompatibility, short production cycle and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
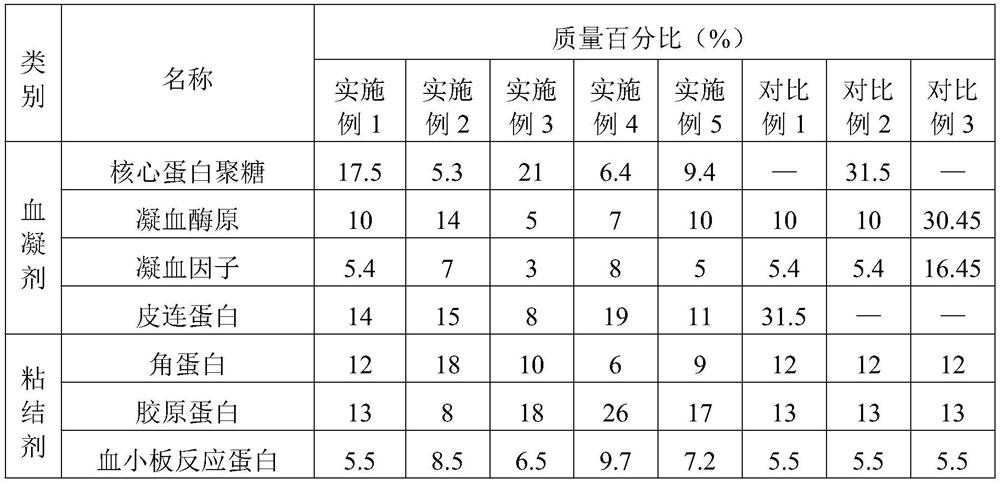
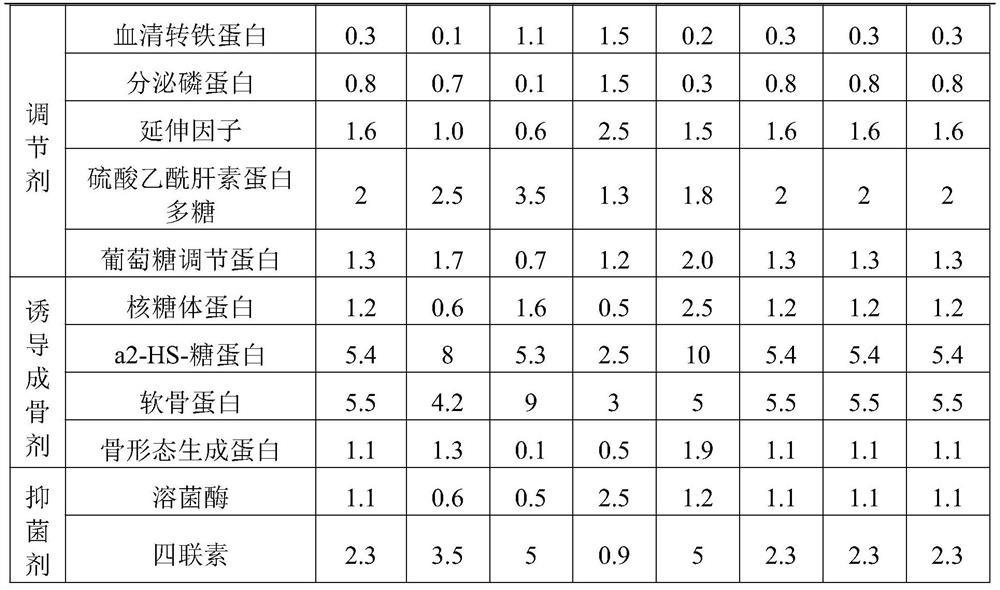
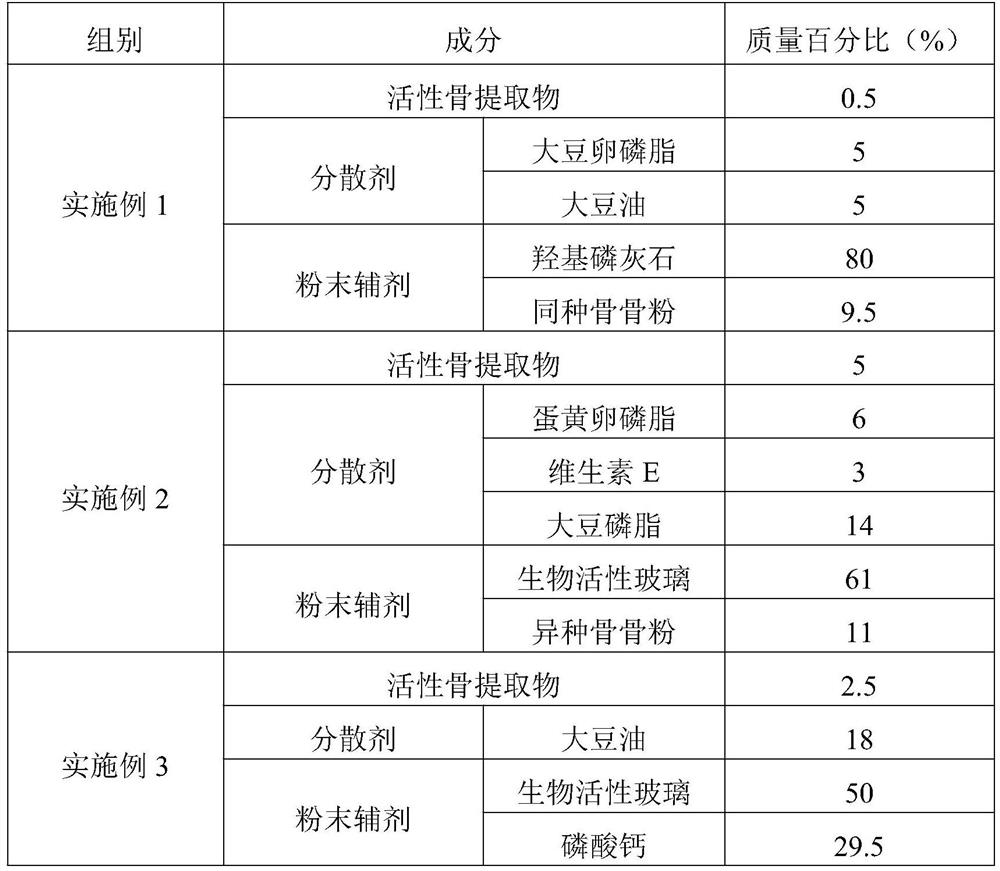
Embodiment 1
[0053] A method for preparing a bone hemostatic material with osteogenic activity, comprising the steps of:
[0054] (1) Grinding: use a grinder to grind the cortical bone, and use liquid nitrogen to keep the temperature low during the grinding process. The cortical bone blocks were crushed into 100-6 mesh cortical bone particles.
[0055] (2) Degreasing: The degreasing solution is a mixture of chloroform / methanol with a volume ratio of 1:1. Add 0.1 times of methanol and 0.2 times of chloroform to the reaction tank in turn, and react at room temperature After 12 hours, replace the degreasing solution and degrease again.
[0056] (3) Decalcification: Prepare a decalcification solution (0.6mol / L hydrochloric acid), soak the cortical bone particles in the decalcification solution, and the decalcification solution just submerges the cortical bone particles. The reaction was carried out at room temperature for 26 hours. Replace the decalcification solution and decalcify twice ag...
Embodiment 2
[0063] A method for preparing a bone hemostatic material with osteogenic activity, comprising the steps of:
[0064] (1) Grinding: use a grinder to grind the cortical bone, and use liquid nitrogen to keep the temperature low during the grinding process. The cortical bone blocks were crushed into 100-6 mesh cortical bone particles.
[0065] (2) Degreasing: The degreasing solution is a mixture of chloroform / methanol with a volume ratio of 1:1. Add 0.3 times of methanol and 0.7 times of chloroform to the reaction tank in turn, and react at room temperature After 16 hours, replace the degreasing solution and degrease again.
[0066] (3) Decalcification: prepare a decalcification solution (0.5mol / L hydrochloric acid), soak the cortical bone particles in the decalcification solution, and the decalcification solution just covers the cortical bone particles. The reaction was carried out at room temperature for 28 hours. Replace the decalcification solution and decalcify twice again...
Embodiment 3
[0073] A method for preparing a bone hemostatic material with osteogenic activity, comprising the steps of:
[0074] (1) Grinding: use a grinder to grind the cortical bone, and use liquid nitrogen to keep the temperature low during the grinding process. The cortical bone blocks were crushed into 100-6 mesh cortical bone particles.
[0075] (2) Degreasing: The degreasing solution is a mixture of chloroform / methanol with a volume ratio of 1:1. Add methanol 0.5 times the weight of cortical bone grains and chloroform 1.5 times the weight of the cortical bone into the reaction tank in turn, and react at room temperature After 18 hours, replace the degreasing solution and degrease again.
[0076] (3) Decalcification: Prepare a decalcification solution (0.4mol / L hydrochloric acid), soak the cortical bone particles in the decalcification solution, and the decalcification solution just covers the cortical bone particles. The reaction was carried out at room temperature for 28 hours. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com