Boron-doped and phosphite-coated nickel-based positive electrode material for lithium ion all-solid-state battery and preparation method of boron-doped and phosphite-coated nickel-based positive electrode material
A technology of all-solid-state batteries and lithium-ion batteries, which is applied in phosphorous acid, battery electrodes, positive electrodes, etc., can solve the problems of low electronic conductance without too much attention, and achieve good lithium ion conductivity and good application Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
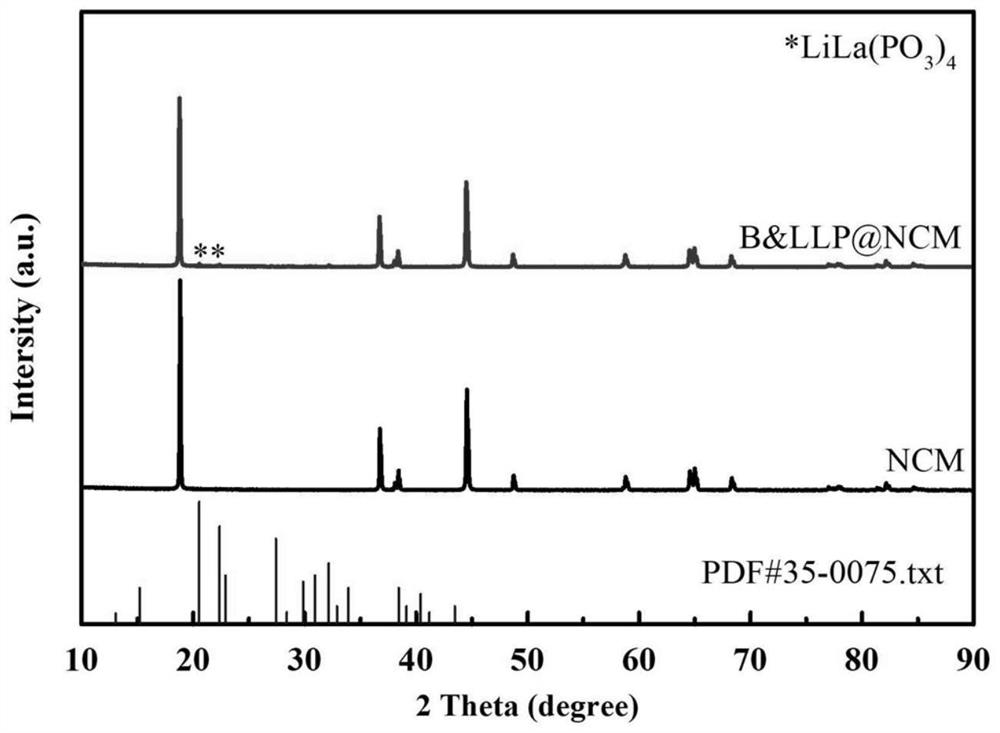
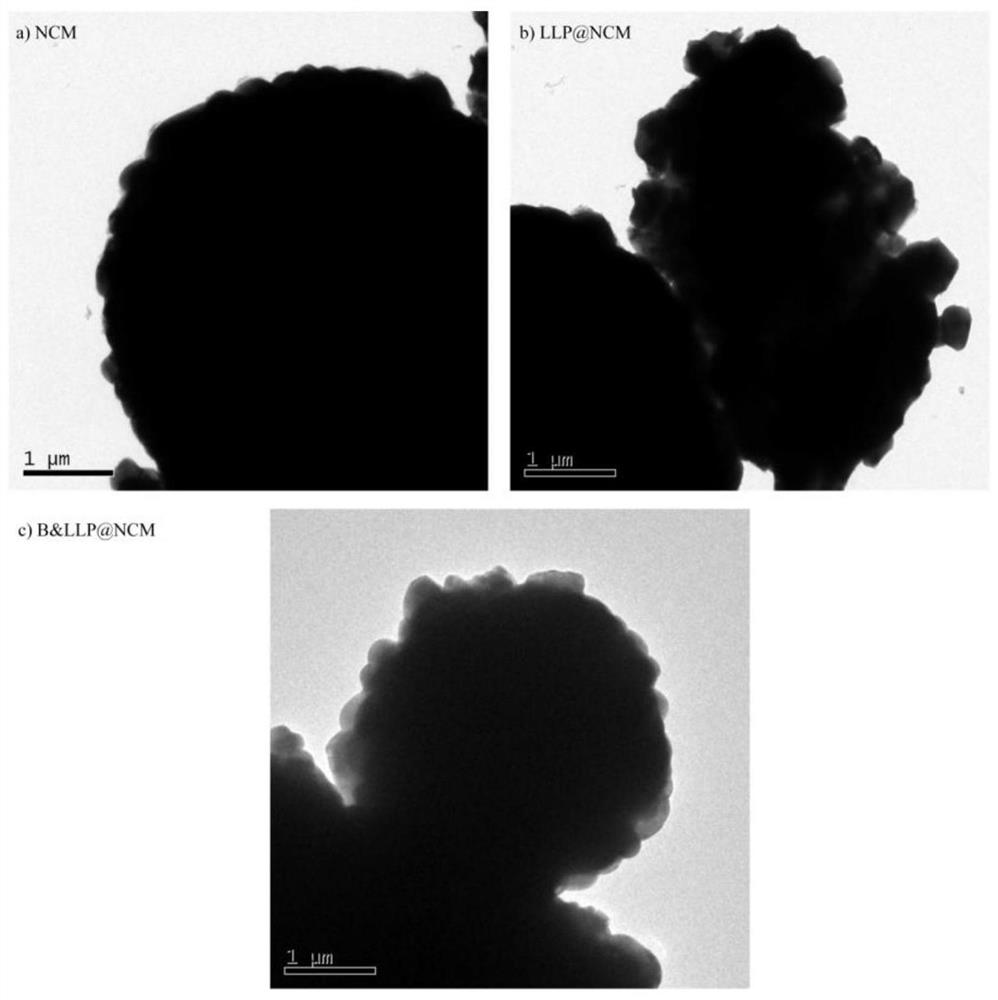
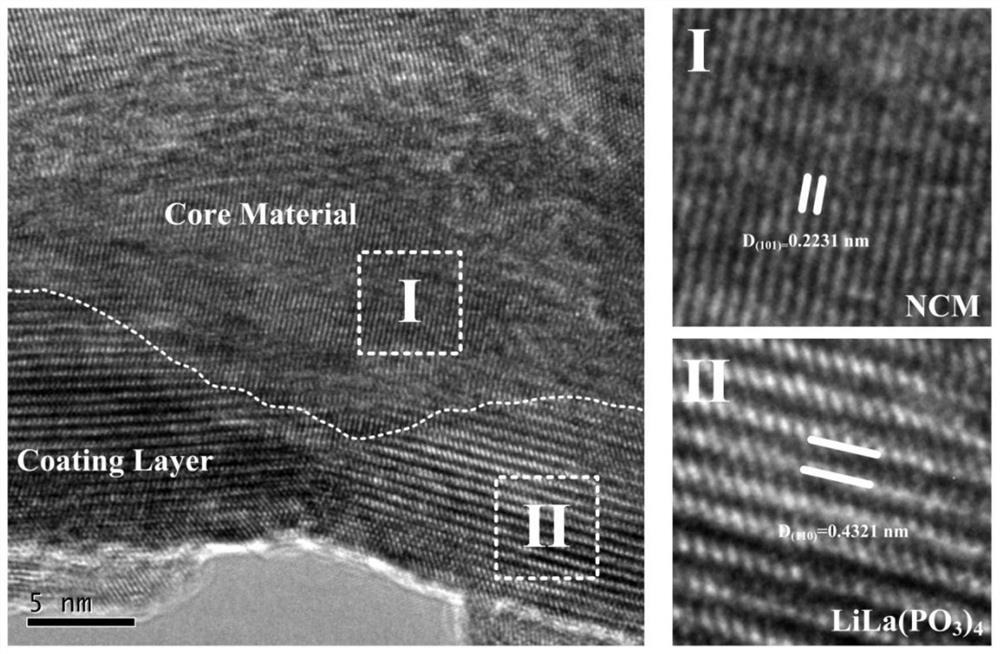
Image
Examples
Embodiment 1
[0039] Weigh 0.0024mol of ammonium hydrogen phosphate, 0.0006mol of lanthanum hydroxide, 0.0324mol of lithium hydroxide and 0.0003mol of boric acid, respectively, and add them to a 250mL ball mill jar containing 20mL of ethylene glycol, in a ratio of 4:3:3 by mass. Add a total of 200g of zirconia grinding balls with particle sizes of 8mm, 12mm and 15mm, place the ball mill on a ball mill and grind for 3h at a speed of 500rmp, and then add 0.03mol of Ni 0.7 Co 0.15 Mn 0.15 (OH) 2 The precursor was continuously ball-milled at a speed of 100 rpm for 2 hours, and the uniformly stirred mixture was taken out and dried in a blast drying oven at 90° C. for 24 hours. The product obtained after drying was placed in a mortar and evenly ground, and then passed through a 400-mesh sieve. The powder was then calcined at 450 °C for 6 h in a tube furnace, heated to 800 °C for 15 h, and boron-doped &LiLa(PO) was obtained. 3 ) 4 (LLP) coated LiNi 0.7 Co 0.15 Mn 0.15 O 2 positive electrod...
Embodiment 2
[0045] Weigh 0.003mol of ammonium dihydrogen phosphate, 0.0004mol of lanthanum oxide, 0.324mol of lithium hydroxide and 0.003mol of boron oxide, respectively, and add them into a 500mL ball mill jar containing 200mL of propanol, according to the mass ratio of 4:3:3. A total of 200 g of zirconia grinding balls with particle sizes of 8 mm, 12 mm and 15 mm were added in proportion, and the ball mill was placed on the ball mill for 3 hours at a speed of 500 rpm, and then 0.3 mol of Ni was added. 0.8 Co 0.1 Mn 0.1 (OH) 2 The precursor was continuously ball-milled at a speed of 100 rpm for 2 hours, and the uniformly stirred mixture was taken out and dried in a blast drying oven at 90° C. for 24 hours. The product obtained after drying was placed in a mortar and evenly ground, and then passed through a 400-mesh sieve. The powder was then calcined at 450 °C for 6 hours in a tube furnace, and then heated to 750 °C and calcined for 15 hours to obtain boron-doped & LLP coating. LiNi ...
Embodiment 3
[0047] Weigh 0.012mol of phosphoric acid, 0.0015mol of lanthanum oxide, 0.165mol of lithium hydroxide and 0.0015mol of boron oxide, respectively, and add them to a 1000mL ball mill jar containing 200mL of propanol. A total of 1000 g of zirconia grinding balls with diameters of 6 mm, 10 mm and 13 mm were placed on the ball mill for 30 h at a speed of 300 rpm, and then 0.15 mol of Ni was added. 0.825 Co 0.115 Al 0.06 O 2 (OH) 2 The precursor was continuously ball-milled at a speed of 300 rpm for 3 hours, and the uniformly stirred mixture was taken out and dried in a blast drying oven at 100° C. for 24 hours. The product obtained after drying was placed in a mortar and evenly ground, then passed through a 500-mesh sieve, and then the powder was calcined at 450 °C for 6 h in a tube furnace, heated to 740 °C and calcined for 12 h to obtain boron-doped & LLP coating. LiNi 0.825 Co 0.115 Al 0.06 O 2 positive electrode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com