Method for selectively modifying cysteine through propargyl type sulfonium salt
A cysteine and propargyl-type technology, applied in the field of biochemistry, can solve the problems of cumbersome modification steps of polypeptides and proteins, and achieve remarkable technological progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
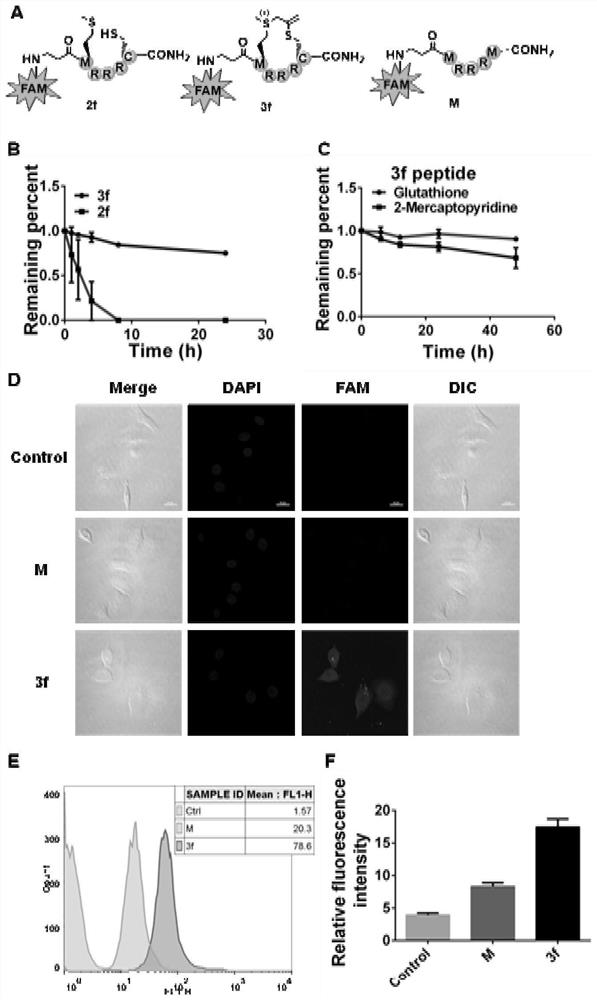
[0038] Solid-phase synthesis and separation and purification of peptides: Weigh Rink amide MBHA resin into a peptide tube, add dichloromethane (DCM), and swell with nitrogen gas for 10 minutes. Add 50% (v / v) morpholine in N,N-dimethylformamide (DMF) solution, blow nitrogen gas for 30 minutes, and remove the Fmoc protecting group. After washing the resin alternately with DMF and DCM, the prepared Fmoc-AA-OH (5eq, 0.4M, DMF) solution, 6-chlorobenzotriazole-1,1,3,3-tetramethylurea Fluorophosphate ester (HCTU) (5eq, 0.38M, DMF) solution and N,N-diisopropylethylamine (DIPEA) (10eq) were mixed well, then added to the resin and blown with nitrogen for 1 hour. Remove the reaction solution, wash the resin according to the above method, continue to deprotect, and connect amino acids until the peptide assembly is completed. Cut the peptide from the resin: take 20mg resin and EP tube, add 0.5ml TFA / TIPS / H 2 O / EDT (v:v:v:v=94:1:2.5:2.5) the shear solution was shaken and reacted for 1 hou...
Embodiment 2
[0040]
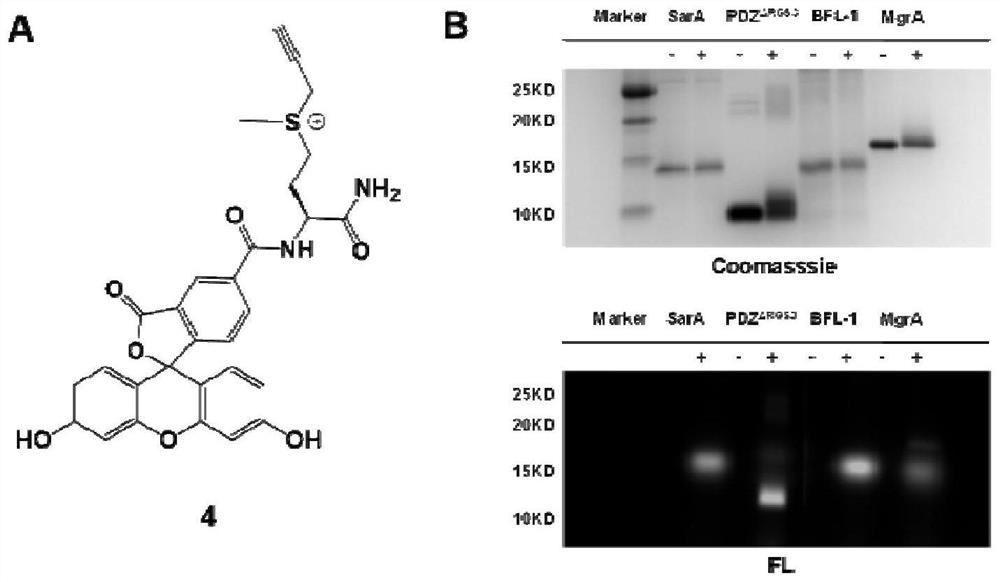
[0041]Synthesis of propargyl-type sulfonium salt: Take a 10mL glass bottle, weigh N-acetyl-L-methionine (0.2mmol, 38.25mg) into the bottle, add 0.2mL of acetonitrile / water (1:1) The solution was acidified by adding 2 μL of formic acid (pH equal to about 3), and propyne bromide (1.0 mmol, 86.2 μL) was added at room temperature, and stirred at room temperature for 12 hours. After the reaction, the solvent was directly spin-dried to obtain a crude product, and the crude product was dissolved in a mixed solution of acetonitrile and water, and then purified by HPLC to obtain a white solid product 1a, 1 HNMR (400MHz,D 2 O)δ4.64-4.55(m,1H),4.40(t,2H),3.53-3.43(m,2H),3.25(td,1H),2.99(s,3H),2.54-2.41(m,1H ),2.35-2.20(m,1H),2.08(s,3H). 13 C{ 1 H}NMR (101MHz,D 2 O) δ174.5, 173.5, 80.8, 51.2, 37.2, 25.6, 25.5, 22.1, 22.0. HRMS (ESI-TOF): m / zcalculated for C 10 h 16 NO 3 S + [M] + ,230.0845,found 230.0848.
[0042] The structures of thiols and products involved in our ...
Embodiment 3
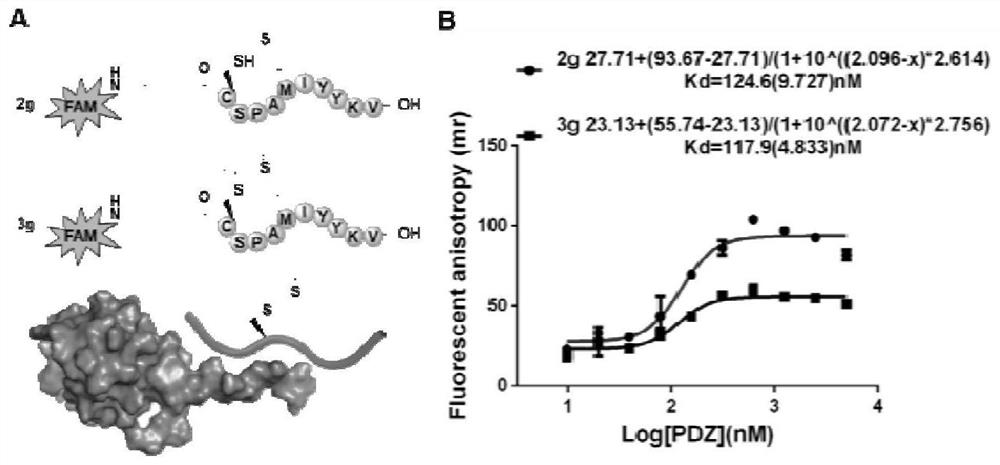
[0047]
[0048] Addition reaction conditions of propargyl sulfonium salt and mercapto group: As mentioned in the table above, the template propargyl sulfonium salt uses different reaction conditions and product structures of sulfhydryl reagents under the experimental conditions. Take a 10 mL glass bottle, weigh N-acetyl-L-cysteine (0.2 mmol, 32.64 mg), add 0.5 mL of ammonium carbonate solution (pH 8.0), and add compound 1a (0.2 mmol, 46.06 mg). React at room temperature for 1 hour, directly spin the solvent to obtain a crude product, add a mixed solution of acetonitrile and water to dissolve the crude product, and purify by HPLC to obtain product 3c. 1 H NMR (400MHz,D 2 O)δ5.24(s,1H),4.98(s,1H),4.28-4.23(m,1H),4.18-4.14(m,1H),3.33-3.08(m,4H),2.95-2.84(m ,1H),2.81-2.74(m,2H),2.71-2.62(m,1H),1.92-1.83(m,9H). 13 C{ 1 H}NMR (101MHz,D 2 O) δ177.0, 176.7, 173.6, 173.6, 173.5, 171.0, 160.3, 113.1, 54.4, 53.9, 38.1, 33.2, 32.9, 22.1, 22.1. HRMS (ESI-TOF): m / z calculated for C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com