Kit for analyzing HER2 gene copy number variation by combining multiple internal references with sequential probability ratio test and use method
A technology of gene copy number and sequential probability ratio, which is applied in the field of biomedical nucleic acid detection, can solve the problem of low accuracy of HER2 gene copy number variation status, and achieve the goal of avoiding subjective errors, reducing the number of samples, and improving accuracy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Establishment of a kit for analyzing HER2 gene copy number variation with multiple internal references combined with SPRT and its application method
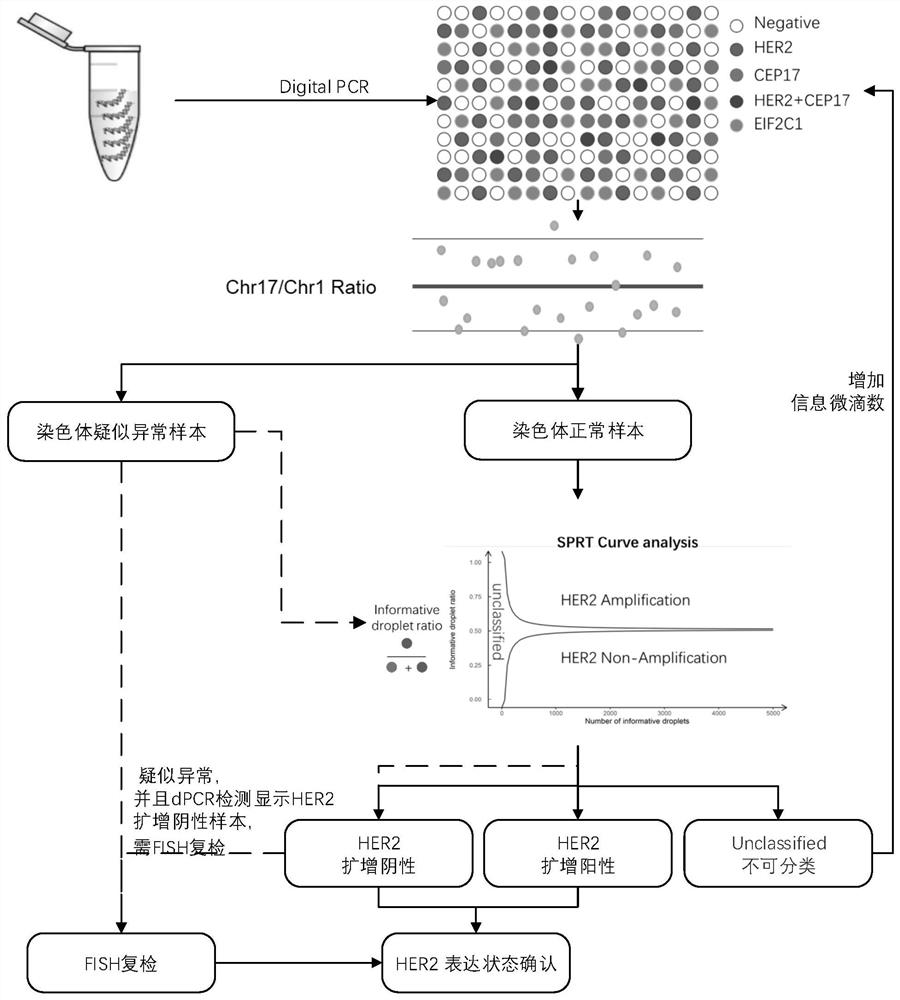
[0024] In this example, a method for detecting HER2 gene copy number variation was established using paraffin samples of breast cancer cell lines, and the detection ability of the method was preliminarily evaluated. Embodiments 1 to 3 are all according to figure 1 The flow chart shown is for the use of the kit and the statistical analysis of subsequent data.
[0025] 1. The nucleotide sequences of the primers and probe nuclei involved in the present invention are shown in Table 1.
[0026] Table 1, the nucleotide sequence list of primers and probes
[0027] serial number name Nucleotide sequence SEQ ID NO: 1 HER2-FPrimer 5'-CTAGCACCTTGCTAAGCA-3' SEQ ID NO: 2 HER2-RPrimer 5'-GAGCACCATTCACAGAAA-3' SEQ ID NO: 3 CEP17-FPrimer 5'-TCTGCCTAATCTACCAATG-3' SEQ ID NO: 4 CE...
Embodiment 2
[0057] Example 2 The state of HER2 gene copy number variation in 10 cases of paraffin tissue section samples was detected using the kit and method of use described in the present invention, and compared with the accuracy of the ratio directly calculated using the CEP17 single internal reference, CEP17 / EIF2C1 The calculated confidence interval and HER2 / CEP17 threshold line are the same as those in Example 1.
[0058] 1. Sample preparation: 10 paraffin samples of breast cancer cell lines.
[0059] 2. The procedures for DNA extraction and digital PCR detection are the same as in Example 1.
[0060] 3. The results of digital PCR detection and SPRT analysis are shown in Table 7 and Table 8.
[0061] Table 7. Detection results of HER2 gene copy number variation status in paraffin samples of 10 breast cancer cell lines using the kit and method of use according to the present invention
[0062]
[0063] Table 8. SPRT analysis results of each sample in 10 breast cancer cell line p...
Embodiment 3
[0068] Example 3 Comparison of the kit and method of use of the present invention with the CEP17 single internal reference analysis method
[0069] 1. Combining the two parts of sample information involved in Example 1 and Example 2, see Table 10 for the comparison results of the kit and usage method of the present invention and the CEP17 single internal reference analysis method.
[0070] Table 10. In 40 cases of breast cancer cell line paraffin samples, the comparison between using the kit and using method of the present invention and CEP17 single internal reference analysis method
[0071]
[0072] 2. After a comprehensive analysis, it was found that in 40 samples, using the CEP17 single internal reference analysis method to detect the status of HER2 gene copy number variation showed that the detection results of samples 26, 34, 35, 37, and 40 were consistent with IHC / FISH results. Conformity, the accuracy rate is 87.5% (35 / 40, 95% CI 73.89%-94.54%), and the status of ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com