Anti-herpes simplex virus type I drug and preparation method and application thereof
A herpes simplex virus and drug technology, applied in the field of biomedicine, can solve unreported problems and achieve good biological safety, good therapeutic effect, and the effect of inhibiting growth and reproduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
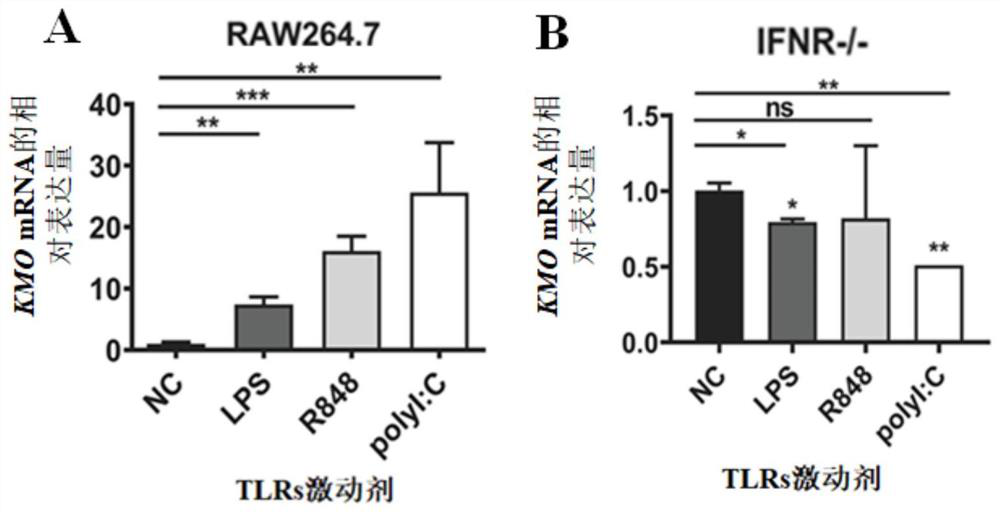
[0065] This example verifies from the gene level that KMO is an interferon-activated gene and has the effect of inhibiting HSV-1.
[0066] In order to verify that KMOs belong to ISGs, different Toll-like receptors (TLRs) agonists were used in this example: LPS (1 μg / mL), R848 (100 nM) and polyI:C (25 μg / mL) to stimulate Raw264.7 and IFN Receptor-deficient (IFNR- / -) J2 BMMs cells were used for 4 hours, and the cell lysate was taken for RT-qPCR detection of KMO gene expression.
[0067] Among them, the methods of RNA isolation, reverse transcription and RT-qPCR detection are as follows:
[0068](1) RNA isolation: Add 1 mL of TROIZOL reagent to the cells, leave at room temperature for 5 minutes, transfer to a 1.5 mL centrifuge tube, add 0.2 mL of chloroform to the centrifuge tube, close the centrifuge tube cap tightly, and swing the centrifuge tube up and down Mix well for 15s, let stand at room temperature for 2min, and centrifuge at 4°C and 12000g for 15min;
[0069] Take 0.5...
Embodiment 2
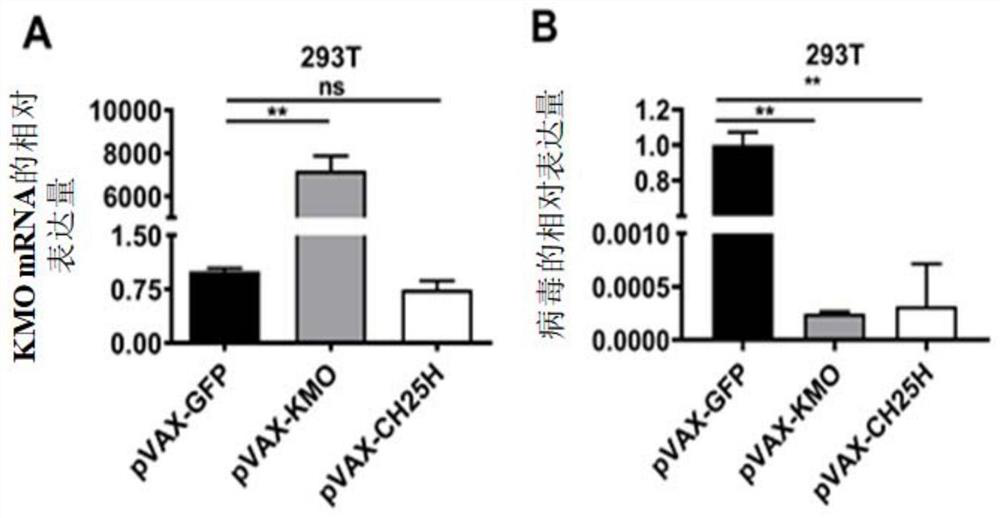
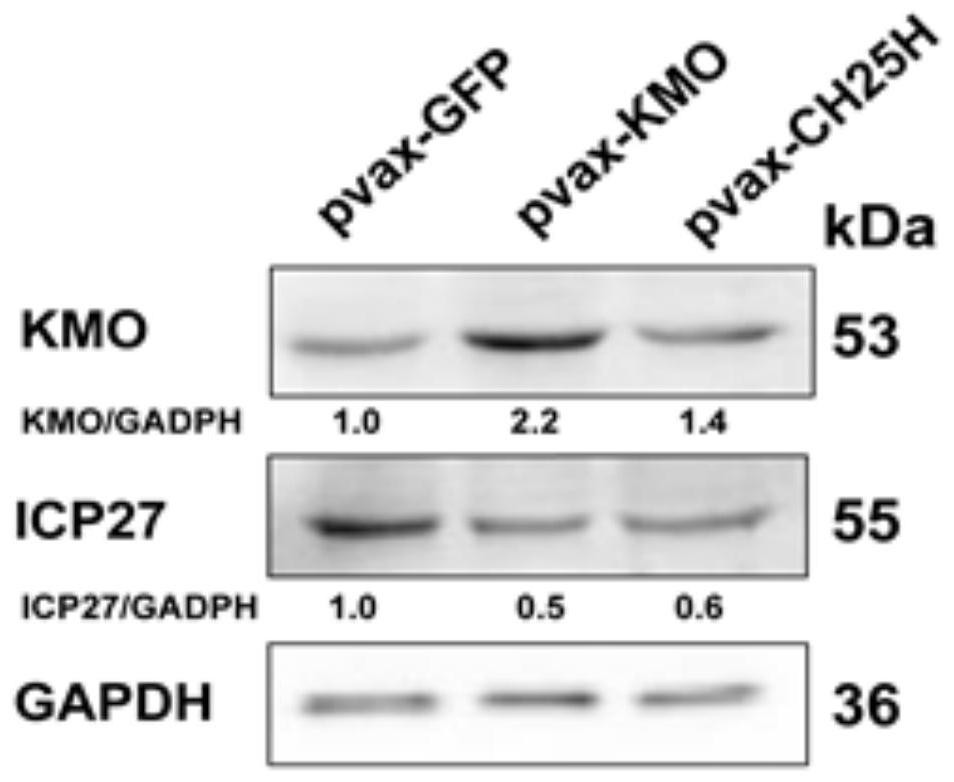
[0084] This example verifies that the overexpression of KMO has an inhibitory effect on HSV-1 infection from the protein and cellular levels. The specific method is as follows:
[0085] (1) Western blotting
[0086] Prepare lysate (RIPA:PMSF:protease inhibitor:phosphatase inhibitor=100:1:1:1); discard the culture medium from the cells in the 6-well plate, add 200 μL of lysate to each well on ice, and place it on ice. Shaker in a refrigerator at 4°C, fully lysed for 20min; use a cell scraper to scrape cells, and transfer them to a centrifuge tube; centrifuge at 12,000g at 4°C for 15min, transfer 160μL of supernatant to a new centrifuge tube; add 40μL of 5× protein loading buffer;
[0087] Seal the cap and heat at 100°C for 10min; use the remaining supernatant to measure the protein concentration by BCA; adjust the protein concentration of each sample to the same level, and store the heat-denatured protein at -20°C;
[0088] Configure 10% SDS-PAGE gel, 60V voltage for 1h, afte...
Embodiment 3
[0099] In this example, the effect of KMO on HSV-1 was studied by down-regulating the expression level of KMO.
[0100] To investigate the effect of KMO downregulation and mutation on HSV-1 replication, small interfering RNA (siRNA) was used in this example to silence the expression of KMO in 293T cells. The specific method is as follows:
[0101] (1) siRNA silencing
[0102] Synthetic siRNA (GenePharma) was transfected into 293T cells with Lipofectamine RNA iMax. RNA samples were collected 48 hours after transfection for RT-qPCR detection. The sequences of all siRNAs are listed in Table 2 and all RT-qPCR primers are listed in Table 1.
[0103] 293T cells were transfected with siRNA lentivirus for 24 hours, and then infected with HSV-1 (0.25MOI) for 8 hours. KMO expression and HSV-1 virus expression were detected by RT-qPCR. Image 6 , where A is the expression level of KMO, and B is the expression level of HSV-1 virus. The expression of KMO was significantly decreased af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com