A method of priority lithium lifting and collaborative manganese from waste lithium -ion batteries
A technology of lithium ion battery and ternary battery is applied in the field of separation and extraction of lithium and synergistic recovery of manganese to achieve the effects of reducing energy consumption, reducing production cost and low roasting temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
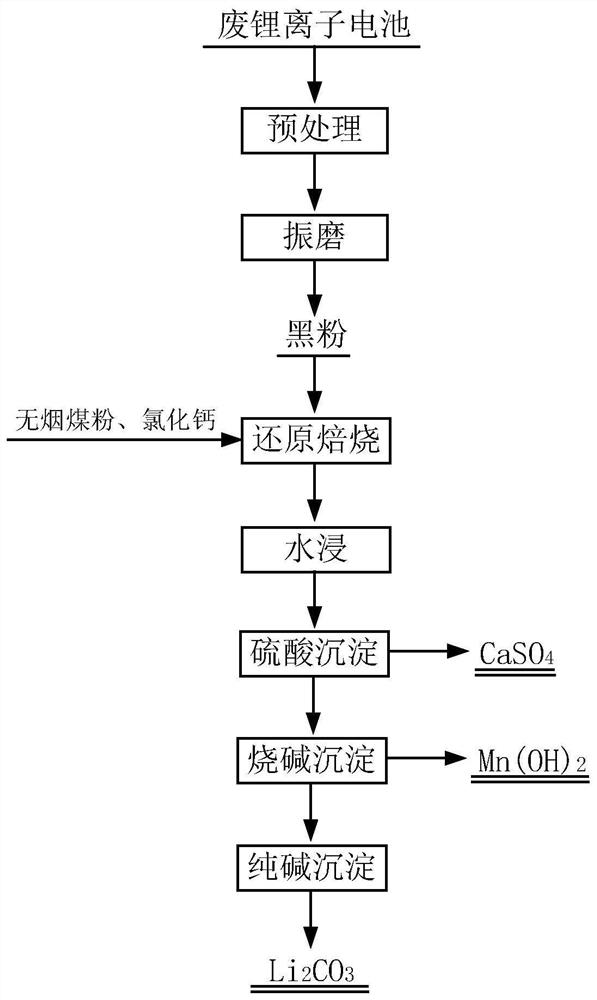
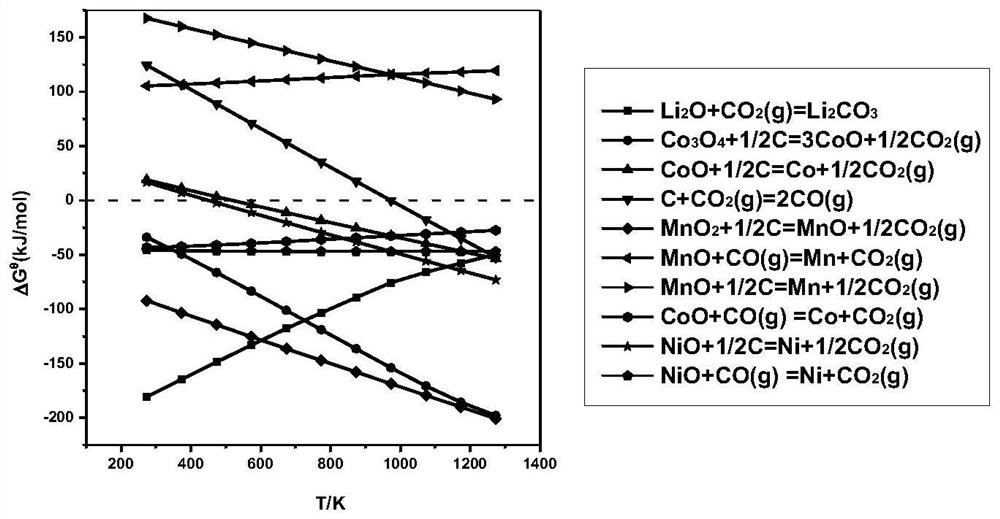
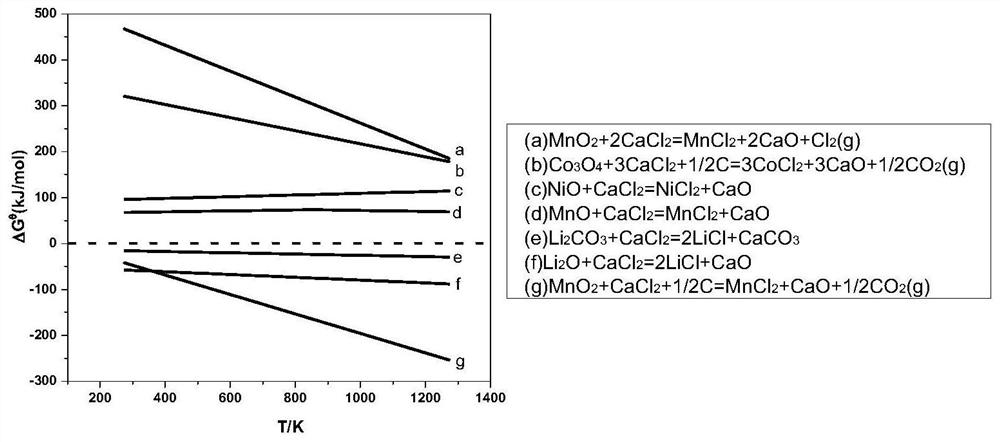
[0047] The waste lithium-ion battery used in this embodiment is a nickel-cobalt-manganate lithium-ion battery. Weigh 30 g of black powder, 12 g of anthracite powder, and 5 g of calcium chloride prepared according to the above steps respectively, mix the three well, pour them into a crucible, and place the crucible in a tube furnace. Use a vacuum pump to evacuate the tube furnace, blow in nitrogen, set the roasting temperature to 650°C, and set the holding time to 2h. After roasting, cool down to room temperature; Leaching, the leaching temperature is 60 °C, the leaching time is 1 h, the leaching solution containing lithium is obtained by filtration, and the filter residue is sent to sulfuric acid leaching to recover Ni, Co and part of Mn; a certain amount of H is added to the leaching solution containing lithium. 2 SO 4 , maintain the pH value at about 2, the reaction temperature is 50 °C, the reaction time is 1 h, and the filtration is separated to obtain CaSO 4 By-product;...
Embodiment 2
[0049] The waste lithium ion battery used in this embodiment is a lithium nickelate battery. Weigh 30 g of black powder, 10 g of anthracite powder, and 5 g of calcium chloride prepared according to the above steps, mix the three well, pour them into a crucible, and place the crucible in a tube furnace. Use a vacuum pump to evacuate the tube furnace, blow in nitrogen, set the roasting temperature to 650°C, and set the holding time to 2h. After roasting, cool down to room temperature; Leaching, the leaching temperature is 60 °C, the leaching time is 1 h, the leaching solution containing lithium is obtained by filtration, and the filter residue is sent to sulfuric acid leaching to recover Ni; a certain amount of H is added to the leaching solution containing lithium. 2 SO 4 , maintain the pH value at about 3, the reaction temperature is 50 ° C, the reaction time is 1 h, and the filtration is separated to obtain CaSO 4 The filtrate is sent to the next step; the filtrate is added...
Embodiment 3
[0051] The waste lithium ion battery used in this embodiment is a lithium cobalt oxide battery. Weigh 30 g of black powder, 10 g of anthracite powder, and 5 g of calcium chloride prepared according to the above steps, mix the three well, pour them into a crucible, and place the crucible in a tube furnace. Use a vacuum pump to evacuate the tube furnace, blow in nitrogen, set the roasting temperature to 650°C, and set the holding time to 2h. After roasting, cool down to room temperature; Leaching, the leaching temperature is 60 °C, the leaching time is 1 h, the leaching solution containing lithium is obtained by filtration, and the filter residue is sent to sulfuric acid leaching to recover Co; a certain amount of H is added to the leaching solution containing lithium. 2 SO 4 , maintain the pH value at about 3, the reaction temperature is 50 ° C, the reaction time is 1 h, and the filtration is separated to obtain CaSO 4 The filtrate is sent to the next step; the filtrate is ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com