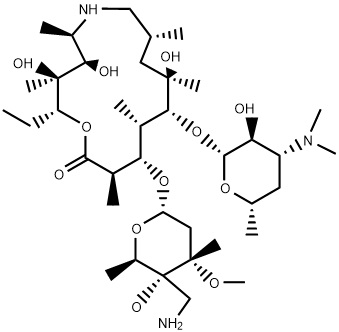
Method for separating and purifying tulathromycin D
A telamycin, separation and purification technology, applied in the field of separation and purification of telamycin D, can solve the problems of incomplete separation, low purity of the target product, incomplete reaction, etc., to achieve improved purity and stable quality of production batches Reliable, short lead time results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
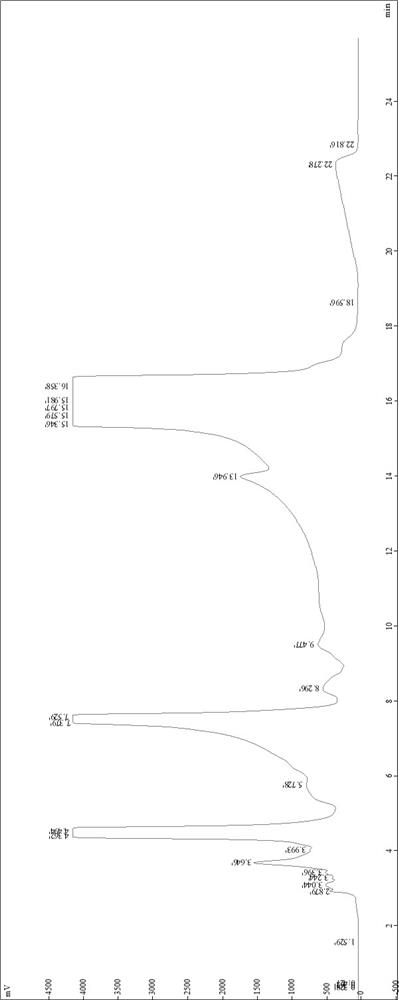
[0052] Chromatographic parameters: C18, 5um filler packing 250g, mobile phase A is 0.01mol / L trisodium phosphate aqueous solution, mobile phase B is acetonitrile and methanol, trisodium phosphate aqueous solution: mobile phase B=40:60, for isocratic elution, The ultraviolet absorption wavelength is 205nm, and the flow rate is 60ml / min.
[0053] The specific process steps are as follows: first dissolve the to-be-separated material, then soak the ion exchange fiber as the separation material in the solution, let it stand for 50 minutes, and then filter out the solution; heat the ammonia water to 65°C, soak the above The ion-exchange fiber that has adsorbed telamycin and telamycin-related substances for 160 minutes was filtered off ammonia; butyl formate was added in an amount 3 times that of the ion-exchange fiber to obtain the pretreated analyte, That is, the treated crude telamycin. Use 10mL of acetonitrile to dissolve 2g of crude telamycin in a neutral environment, filter th...
Embodiment 2
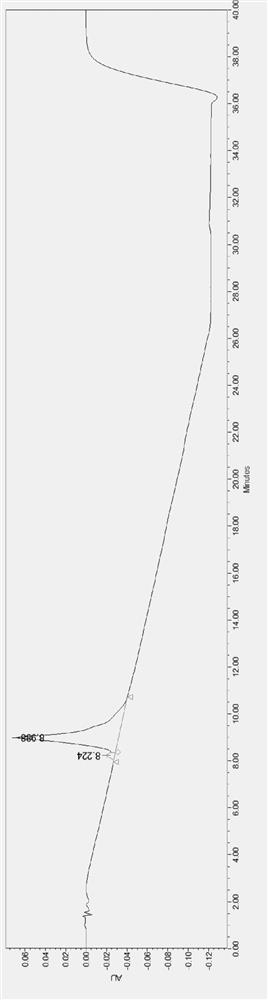
[0055] Chromatographic parameters: C18, 10um filler packing 250g, mobile phase A is 0.01mol / L potassium dihydrogen phosphate aqueous solution, mobile phase B is acetonitrile and ethanol, potassium dihydrogen phosphate solution: mobile phase B=15:85, carry out isocratic washing Desorption, ultraviolet absorption wavelength 205nm, flow rate 60ml / min.
[0056] The specific process steps are as follows: first dissolve the to-be-separated material, then soak the ion-exchange fiber as the separation material in the solution, let it stand for 60 minutes, and then filter out the solution; heat the ammonia water to 61°C, soak the above The ion-exchange fiber that has adsorbed telamycin and telamycin-related substances for 100 minutes was filtered off ammonia; butyl formate was added in an amount 2.5 times that of the ion-exchange fiber to obtain the pretreated analyte, That is, the treated crude telamycin. Use 150mL of acetonitrile to dissolve 1g of crude telamycin in a neutral enviro...
Embodiment 3
[0058] Chromatographic parameters: C8, 10um filler packing 250g, mobile phase A is 0.01mol / L potassium dihydrogen phosphate aqueous solution, mobile phase B is acetonitrile, potassium dihydrogen phosphate aqueous solution: mobile phase B=25:75, for isocratic elution, The ultraviolet absorption wavelength is 205nm, and the flow rate is 60ml / min.
[0059] The specific process steps are as follows: firstly dissolve the material to be separated, then soak the ion exchange fiber as the separation material in the solution, let it stand for 70 minutes, and then filter out the solution; heat the ammonia water to 60°C, take the ammonia water and soak the above The ion-exchange fiber that has adsorbed telamycin and telamycin-related substances for 80 minutes was filtered off ammonia; butyl formate was added in an amount twice that of the ion-exchange fiber to obtain the pretreated analyte, That is, the treated crude telamycin. Use 5 mL of acetonitrile to dissolve 1 g of crude telamycin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com