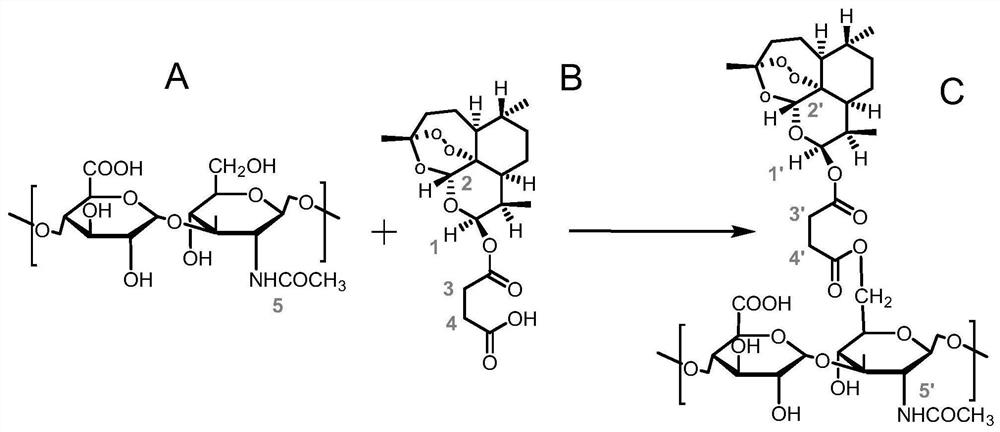
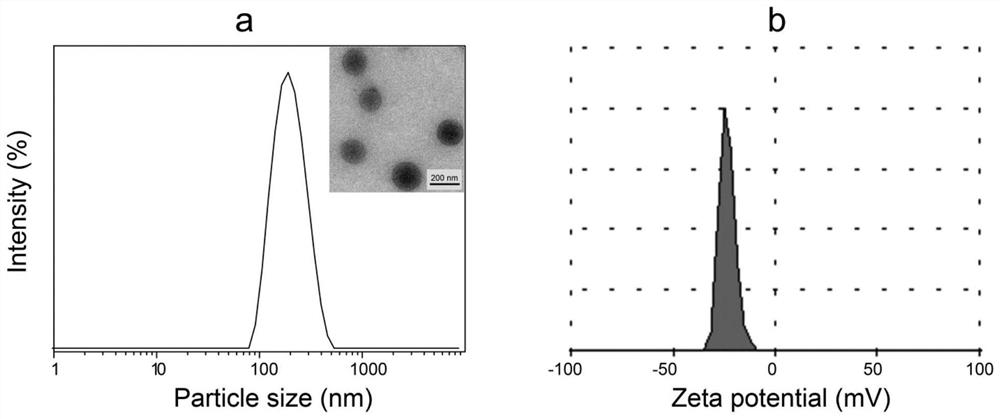
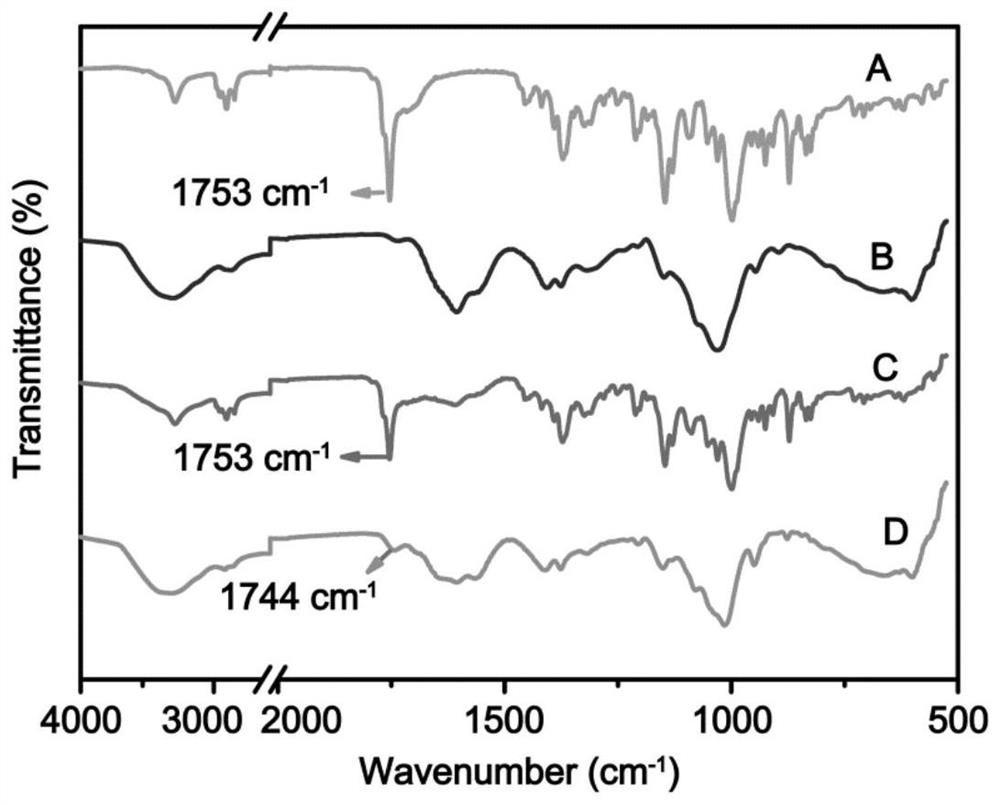
Hyaluronic acid artesunate, nano-micelle preparation of hyaluronic acid artesunate, and preparation method and use of nano-micelle preparation
A technology of artesunate and nano micelles, applied in nanotechnology, nanotechnology, nanomedicine, etc., to achieve the effects of good responsive release characteristics, strong tumor cytotoxicity, and strong targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Dissolve ASA in dimethyl sulfoxide, add the activator EDC·HCl according to the molar ratio of ASA and activator as 1:2, carry out carboxyl activation at 45°C for 0.5h under the protection of nitrogen, and then add The formamide solution of HA, the molar ratio of ASA to HA is 1:2, and the esterification reaction is carried out at 45°C for 48h under the protection of nitrogen;
[0049](2) Dialyzing the material obtained in step (1) to obtain HA-ASA dialyzate;
[0050] (3) After the HA-ASA dialysate obtained in step (2) is vacuum freeze-dried, the corresponding HA-ASA raw material is obtained;
[0051] (4) Dissolving the HA-ASA raw material obtained in step (3) in dimethyl sulfoxide to obtain an organic solution of HA-ASA, and ultrasonically dispersing at room temperature for 0.5 h;
[0052] (5) The organic solution of HA-ASA obtained in step (4) was slowly added to 9 times the volume of deionized water, placed on a shaking table at room temperature for 3 hours, and t...
Embodiment 2
[0056] (1) Dissolve ASA in dimethyl sulfoxide, add the activator EDC·HCl according to the molar ratio of ASA and activator at a ratio of 1:2, carry out carboxyl activation at 50°C for 1 h under nitrogen protection, and then add HA formamide solution, the molar ratio of ASA to HA is 1:2, and the esterification reaction is carried out at 50°C for 36h under the protection of nitrogen;
[0057] (2) Dialyzing the material obtained in step (1) to obtain HA-ASA dialyzate;
[0058] (3) After the HA-ASA dialysate obtained in step (2) is vacuum freeze-dried, the corresponding HA-ASA raw material is obtained;
[0059] (4) dissolving the HA-ASA raw material obtained in step (3) in dimethyl sulfoxide to obtain an organic solution of HA-ASA; ultrasonically disperse at room temperature for 1 h;
[0060] (5) Slowly add the organic solution of HA-ASA obtained in step (4) to 9 times the volume of deionized water, place it on a shaker at room temperature for 1 hour, and then dissolve the HA-ASA...
Embodiment 3
[0064] (1) Dissolve ASA in dimethyl sulfoxide, add the activator EDC·HCI according to the molar ratio of ASA and activator as 1:2, carry out carboxyl activation at 45°C for 2 hours under the protection of nitrogen, and then add HA formamide solution, the molar ratio of ASA to HA is 1:2, and the esterification reaction is carried out at 45°C for 36h under the protection of nitrogen;
[0065] (2) dialyze the material obtained in step (1) to obtain HA-ASA dialysate;
[0066] (3) After the HA-ASA dialysate obtained in step (2) is vacuum freeze-dried, the corresponding HA-ASA raw material is obtained;
[0067] (4) Dissolving the HA-ASA raw material obtained in step (3) in N,N-dimethylformamide to obtain an organic solution of HA-ASA; ultrasonically disperse at room temperature for 0.5h;
[0068] (5) Slowly add the organic solution of HA-ASA obtained in step (4) to 9 times the volume of deionized water, place it on a shaker at room temperature for 3 hours, and then perform dialysis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com