A kind of serine protease inhibitor serpin7 gene of diamondback moth and its application
A protease inhibitor, moth serine technology, which is applied in the field of agricultural biology, can solve the problem of not involving the diamondback moth serine protease inhibitor, etc., and achieves the effect of good biological control potential and application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
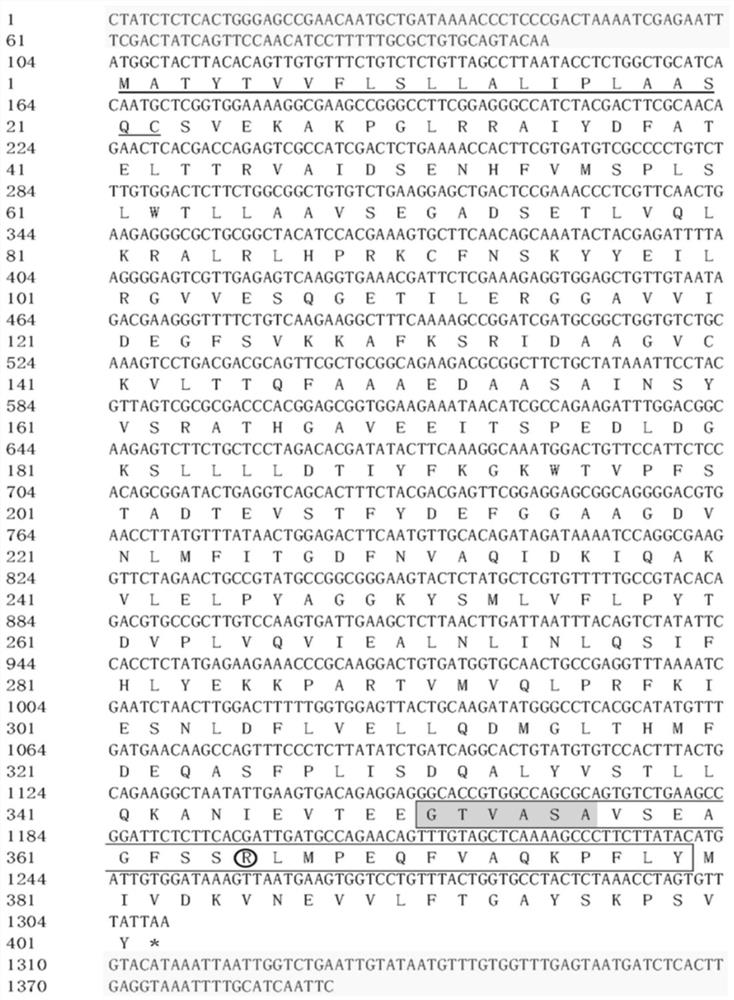
[0040] Cloning and sequence analysis of embodiment 1 Plutella xylostella Serpin7 gene
[0041] 1. Extraction of Plutella xylostella total RNA and synthesis of cDNA first strand
[0042] Take 50-100 mg of the 4th instar larvae of Plutella xylostella xylostella, grind with liquid nitrogen, and then use TRIzol reagent (Invitrogen, USA) to extract total RNA, and the specific operation is carried out according to the instructions.
[0043] The quality and concentration of the extracted RNA were detected. After the standard concentration was determined, the first-strand cDNA was synthesized according to the instructions of the reverse transcription kit (TaKaRa company) and the 5'-cDNA and 3'-cDNA were synthesized according to the instructions of the RACE kit (Clontech company).
[0044] 2. Cloning of the full-length cDNA sequence of Plutella xylostella Serpin7
[0045] Primers Serpin7-F and Serpin7-R were designed according to the Unigene fragment sequence of Serpin7 obtained from ...
Embodiment 2
[0051] Construction of the prokaryotic expression plasmid of embodiment 2 Plutella xylostella Serpin7
[0052] A pair of specific primers were designed according to the ORF (open reading frame) sequence of Plutella xylostella Serpin7 gene (Table 1). In order to facilitate the cloning of the PCR product into the expression vector pET32a, an EcoRI restriction site was designed in the upstream primer, and an XhoI restriction site was designed in the downstream primer. The PCR product was double-digested with EcoRI and XhoI, recovered and purified by tapping the gel, ligated with the expression plasmid pET32a that had been digested with EcoRI / XhoI to construct the prokaryotic expression plasmid pET32a-Serpin7, and transformed into Escherichia coli DH5α, and the sequence was identified by enzyme digestion and sequencing. Accuracy of enzyme cutting sites. The correct monoclonals were identified by enzyme digestion and sequencing, the plasmids were extracted, transformed into expres...
Embodiment 3
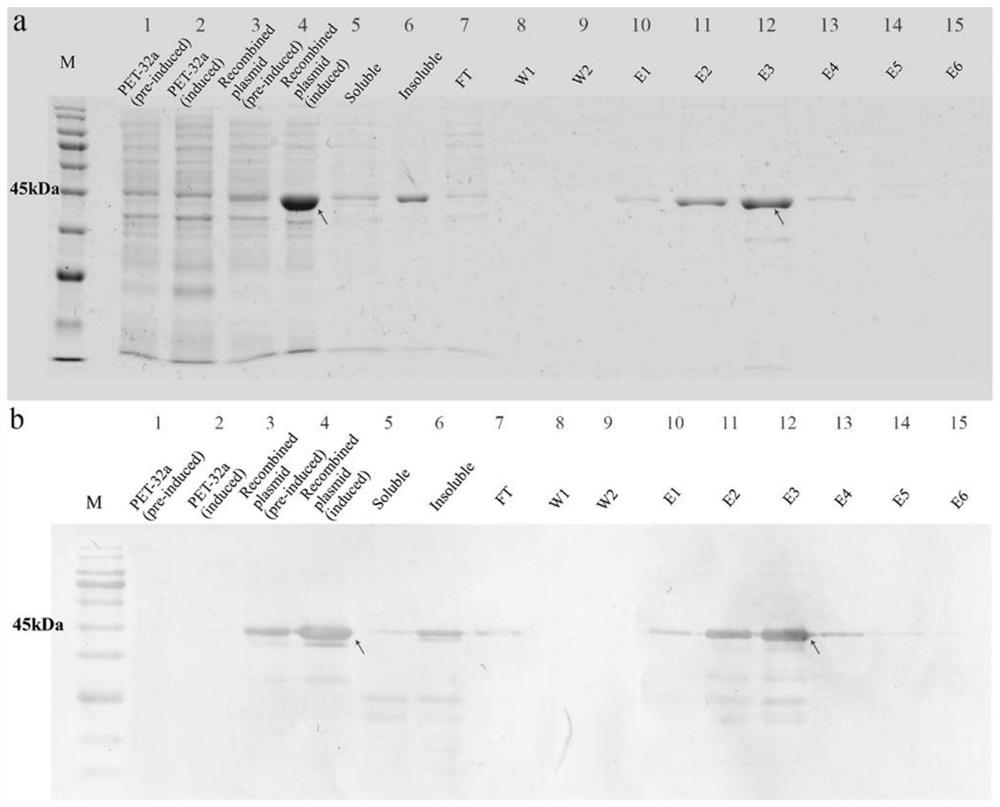
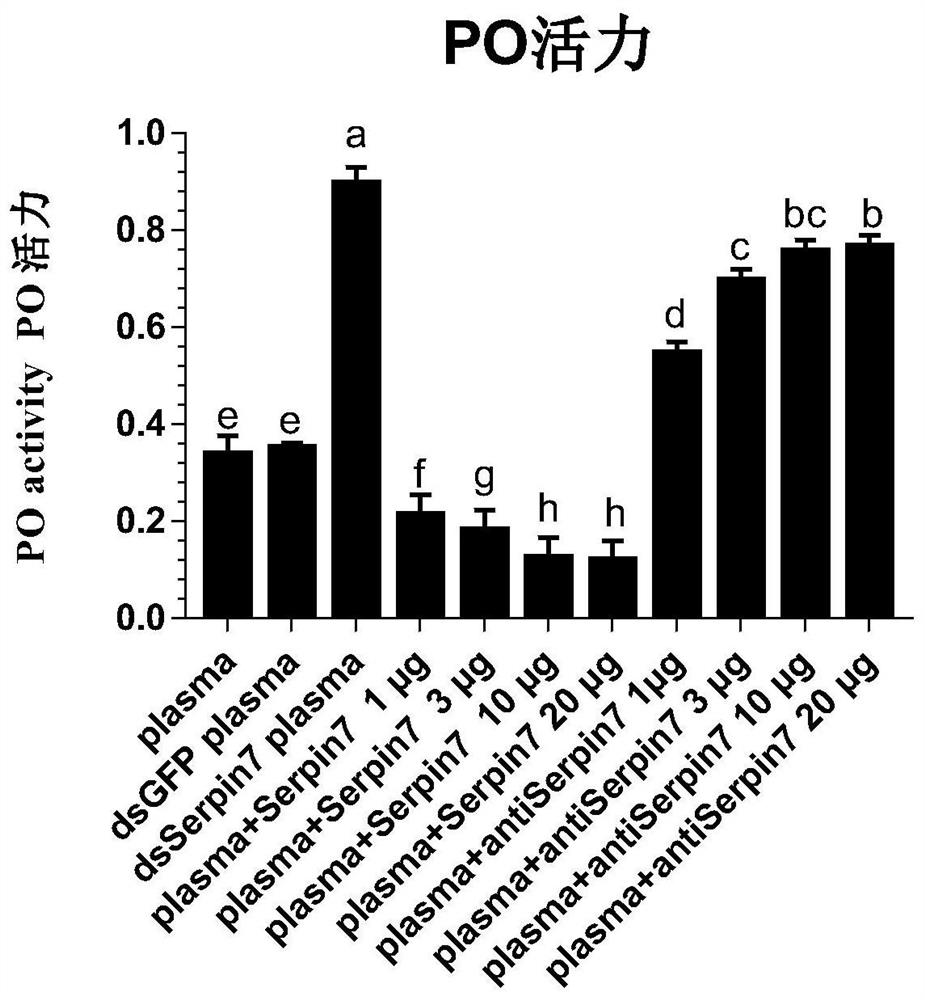
[0053] Example 3 Prokaryotic Expression of Plutella xylostella Serpin7 and Preparation of Polyclonal Antibody
[0054] The single clone was inoculated in 10 mL LB medium (containing 100 μg / mL ampicillin Amp) for overnight culture, and then diluted 1:100 the next day to continue culture at 37 °C, when A 600= 0.6, adding IPTG (isopropylβ-D-1-thiogalactopyranoside) with a final concentration of 0.5mmol / L to induce the expression of the fusion protein. After culturing at 37°C for 4 hours, the culture was collected by centrifugation at 12,000 rpm at 4°C, and the supernatant was discarded. Use bacterial lysate (1.5% sodium lauryl sulfate, 1mM PMSF, 1% TritonX-100, 1mg / mL lysozyme) for the precipitate, lyse on ice for 30min, and then ultrasonicate intermittently until the bacterial solution is clear; 12000rpm, 4°C The supernatant was collected by centrifugation, and the target protein was purified in one step according to Ni-NTA Sefinose Resin (Sangon Biotech). The purification proc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com