Duloxetine pharmaceutical composition
A technology of duloxetine and its composition, which is applied in the field of pharmaceutical preparations, can solve problems such as slow dissolution and lower bioavailability of active compounds, and achieve the effects of reducing reactions, improving stability, and shortening the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation technology of embodiment 1-8 is as follows:
[0043] Step 1: Drug layer – pellet core part
[0044] Duloxetine and Opadry YS-1-7006 (binder), crospovidone and talc were dissolved in purified water. This solution was coated onto sugar sphere cores in a Wurster fluidized bed.
[0045] Step 2: Isolation Layer
[0046] The above drug-loaded pellets were then first coated with a dispersion of sucrose, hypromellose 2910 and purified talc (glidant) in the purification in a Wurster fluidized bed to form coated pellets.
[0047] Step 3: Enteric Coated Layer
[0048] The talc was then purified using hydroxypropylmethylcellulose phthalate (HPMCP) 50 and hydroxypropylmethylcellulose phthalate (HPMCP) 55 in a Wurster fluidized bed (flow aid agent) and diethyl phthalate (plasticizer) in purified water and acetone to coat the above barrier layer pellets to form enteric layered duloxetine pellets.
[0049] Step 4: Outermost Layer
[0050] The enteric-coated layer ...
example 4
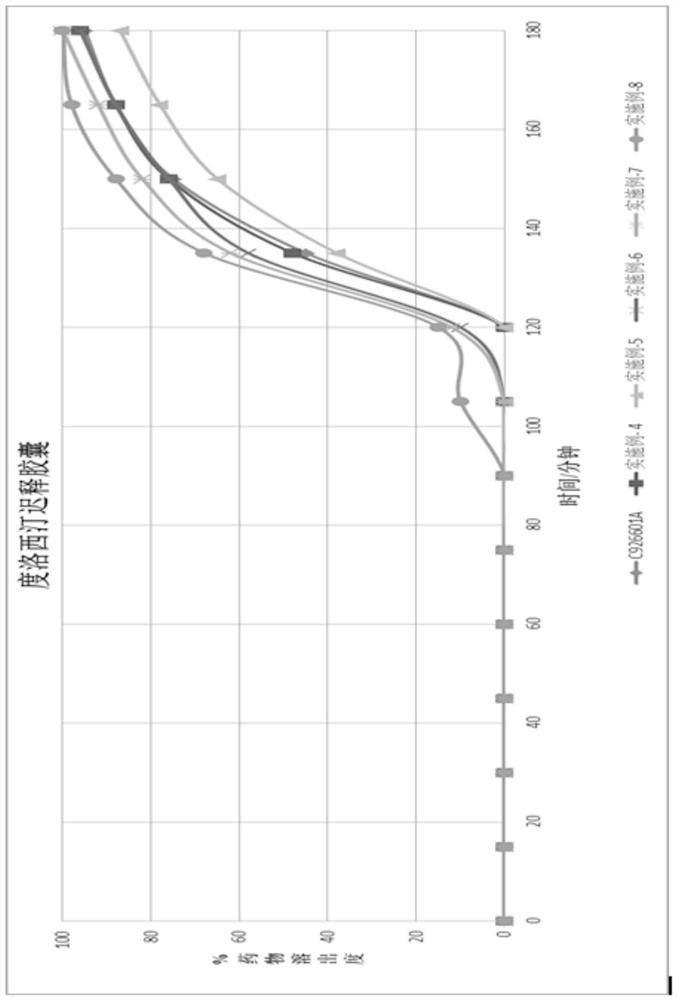
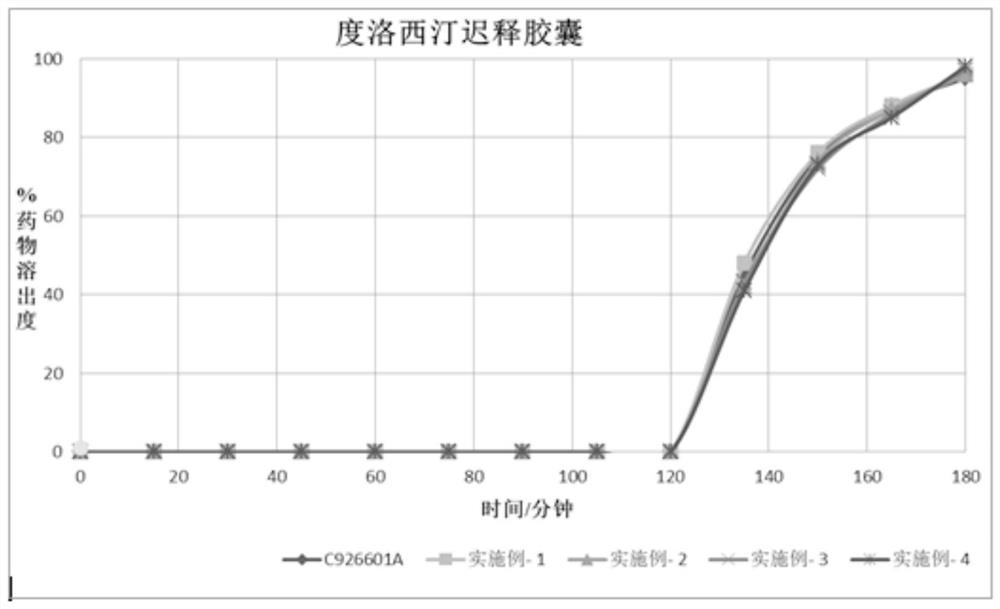
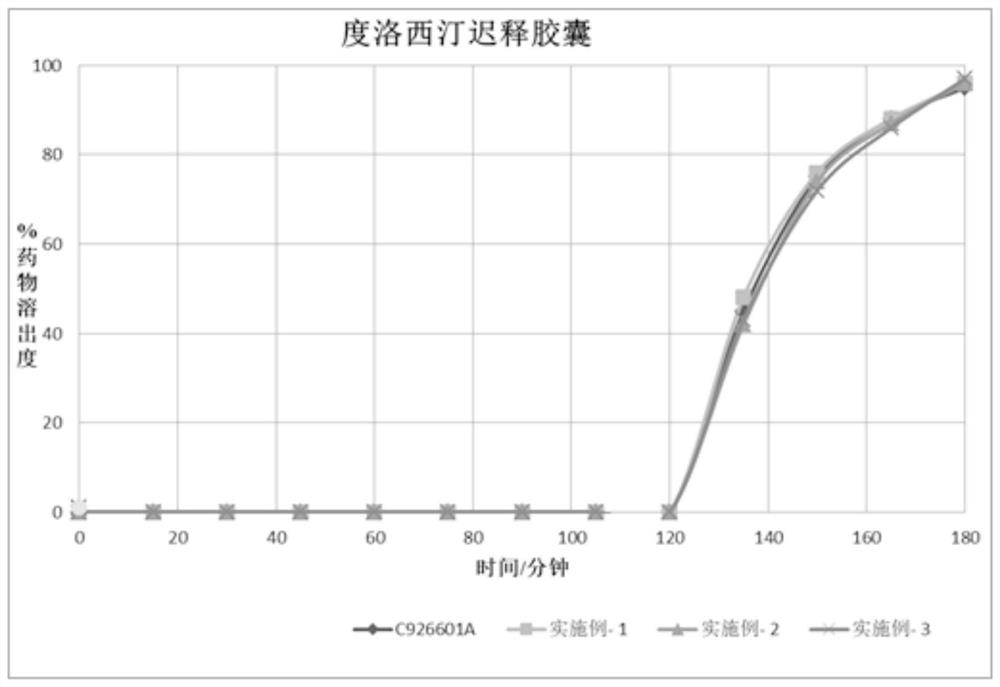
[0058]Example 4 was completely dissolved in the buffer within 1 hour, showing that the in vitro release was similar to that of a well-known brand product (Cymbalta, lot number C926601A).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com