Lipid droplet specifically labeled fluorescent probe and synthesis method and application thereof
A technology of fluorescent probe and synthesis method, which is applied in the field of lipid droplet specific labeled fluorescent probe and its synthesis, can solve the problems to be further developed, and achieves good lipid droplet specific imaging ability, simple operation and simple preparation method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preferred embodiment of the present invention provides a method for synthesizing a lipid droplet-specifically labeled fluorescent probe. The specific steps are as follows:
[0038]
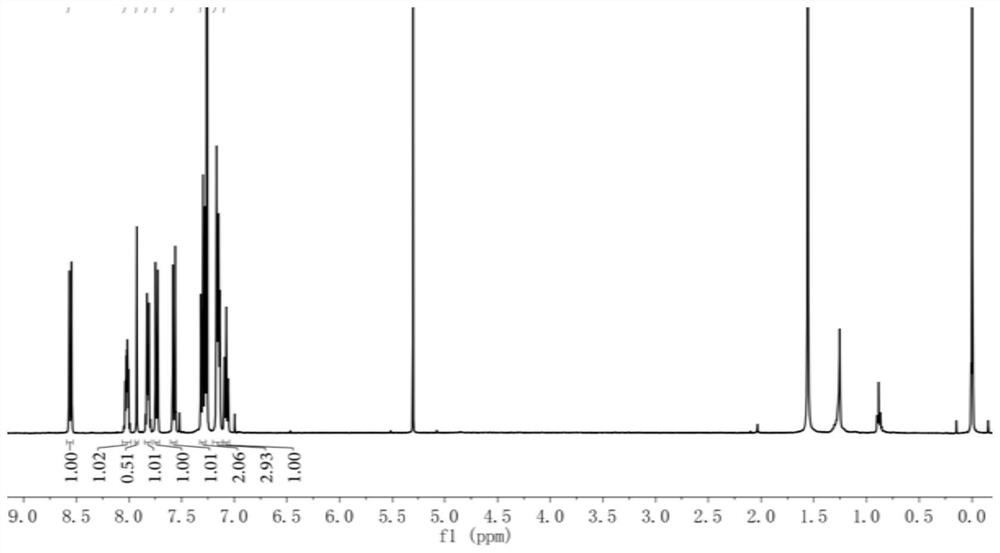
[0039] 4'-(diphenylamino)biphenyl-4-carbaldehyde (0.50g, 1.43mmol), 1,3-indandione (0.23g, 1.58mmol), dissolved in a mixed solution of 5mL methanol and 5mL toluene, Add 2 drops of piperidine, react at 100°C, monitor the reaction process by thin-layer chromatography, cool to room temperature after the reaction, concentrate, and separate and purify the product by silica gel column chromatography to obtain 0.56g reddish-brown solid compound, the yield was 81.96%. 1 H NMR (400MHz, CDCl 3 ):δ=7.05-7.11(t,2H),7.12-7.20(m,6H),7.27-7.33(m,4H),7.55-7.60(d,2H),7.71-7.76(d,2H),7.79 -7.85(m,2H),7.92(s,1H),7.99-8.05(m,2H)ppm.
Embodiment 2
[0041] A preferred embodiment of the present invention provides a method for synthesizing a lipid droplet-specifically labeled fluorescent probe. The specific steps are as follows:
[0042]
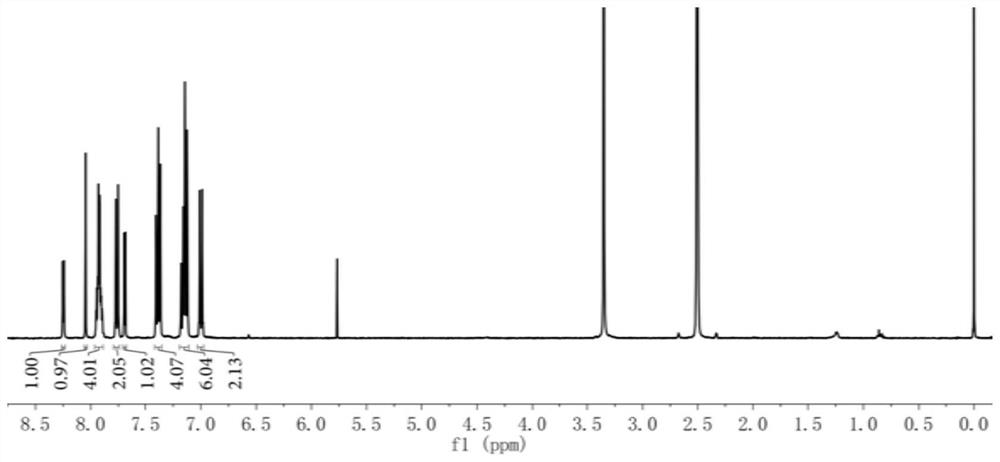
[0043] 5-(4-(diphenylamino)phenyl)thiophene-2-carbaldehyde (0.50g, 1.41mmol), 1,3-indandione (0.22g, 1.51mmol), dissolved in 5mL methanol and 5mL toluene Add 2 drops of piperidine to the mixed solution, react at 120°C, monitor the reaction process by thin-layer chromatography, cool to room temperature after the reaction, concentrate, and separate and purify the product by silica gel column chromatography to obtain 0.58 g of reddish-brown solid compound with a yield of 85.29%. 1 H NMR (400MHz, CDCl 3 ):δ=6.97-7.02(m,2H),7.11-7.19(m,6H),7.35-7.42(t,4H),7.68-7.71(d,1H),7.74-7.79(m,2H),7.88 -7.96(m,4H),8.05(s,1H),8.23-8.27(d,2H)ppm.
Embodiment 3
[0045] A preferred embodiment of the present invention provides a method for synthesizing a lipid droplet-specifically labeled fluorescent probe. The specific steps are as follows:
[0046]
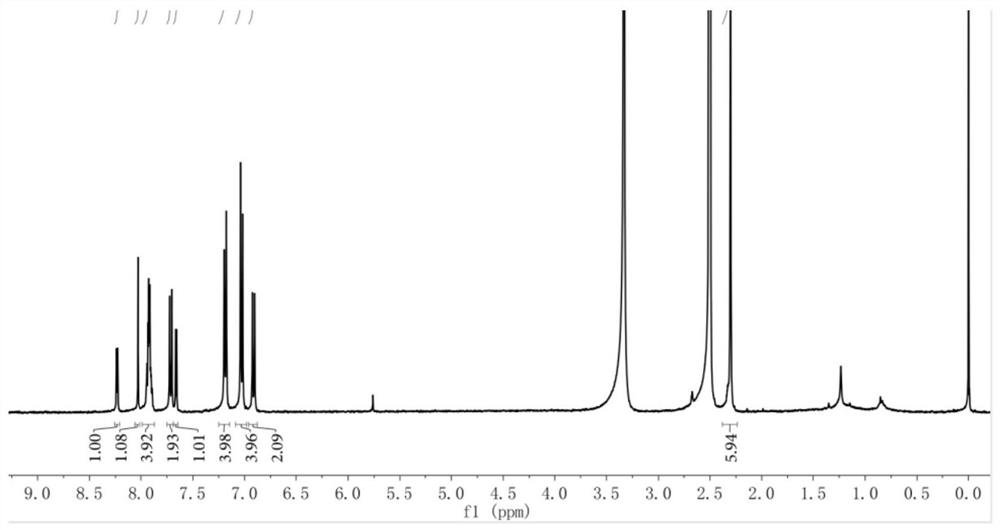
[0047]5-(4-(xylylamino)phenyl)thiophene-2-carbaldehyde (0.60g, 1.56mmol), 1,3-indandione (0.25g, 1.72mmol), dissolved in 10mL of toluene, added 2 drops of piperidine were reacted at 120°C, and the reaction process was monitored by thin-layer chromatography. After the reaction was completed, it was cooled to room temperature, concentrated, and the product was separated and purified by silica gel column chromatography to obtain 0.60 g of a reddish-brown solid compound. The yield was 75.01%. 1 H NMR (400MHz, DMSO-d 6 ):δ=2.30(s,6H),6.90-6.94(d,2H),7.01-7.06(d,4H),7.25-7.23(d,4H),7.64-7.68(d,1H),7.68-7.74 (d,2H),7.87-7.97(m,4H),8.03(s,1H),8.22-8.26(d,2H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com