Polyglycerol fatty acid ester derivative containing polysialic acid group as well as synthesis method and application of polyglycerol fatty acid ester derivative in pharmaceutical preparations
A technology of polysialic acid and fatty acid ester, applied in the field of medicine, can solve problems such as decreased activity, achieve the effects of good biocompatibility and biodegradability, good clinical transformation and industrial development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
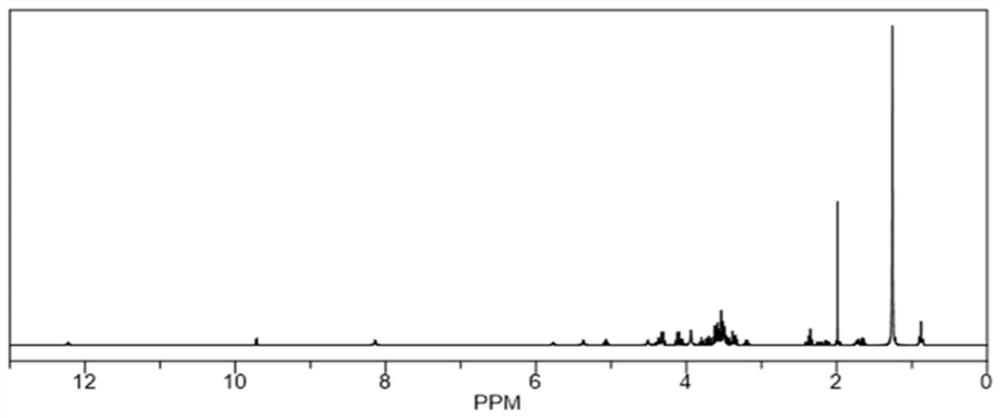
[0035] The synthesis of embodiment 1 polysialic acid-ten polyglycerol distearate
[0036] PSA-PG preparation process of the present invention: at first, feed ratio is polysialic acid (PSA) / decaglyceryl distearate (PG 10 -2C 18 ) / N-hydroxysuccinimide (NHS) / 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) / triethylamine (TEA)=1: 1:2:4:4 (molar ratio); Dissolve an appropriate amount of PSA in 5mL of formamide (FA), add EDC / NHS in proportion, activate at 4°C for 90min, the system is clear at this time; then dissolve in 2mL of FA PG in 10 -2C 18 Add to the reaction system, add TEA, stir at room temperature for 48h, the reaction solution is clear; then transfer the reaction solution to a dialysis bag (molecular weight cut-off 10kDa), the dialysis medium is concentrated hydrochloric acid-water (V / V, 1:100) system, The volume of dialysis was 1000mL, and the dialysis medium was changed every 4 hours for a total of 24 hours of dialysis; finally, part of the water was r...
Embodiment 2
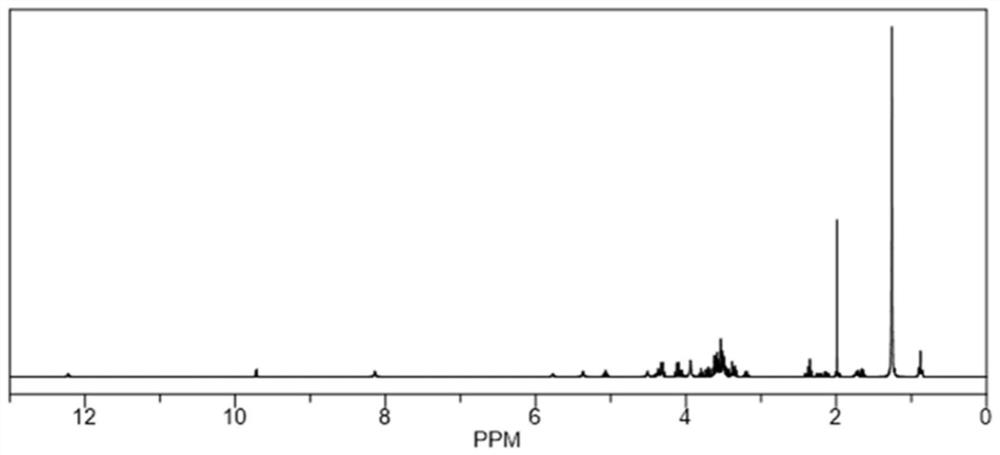
[0044] The synthesis of embodiment 2 polysialic acid-dodepolyglycerol dipalmitate
[0045] The PSA-PG preparation process of the present invention: at first, feed ratio is polysialic acid (PSA) / dodecyl glyceryl dipalmitate (PG 12 -2C 16 ) / N-hydroxysuccinimide (NHS) / 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) / triethylamine (TEA)=1: 1:2:4:4 (molar ratio); Dissolve an appropriate amount of PSA in 5mL of formamide (FA), add EDC / NHS in proportion, activate at 4°C for 90min, the system is clear at this time; then dissolve in 2mL of FA PG in 12 -2C 16 Add to the reaction system, add TEA, stir at room temperature for 48h, the reaction solution is clear; then transfer the reaction solution to a dialysis bag (molecular weight cut-off 10kDa), the dialysis medium is concentrated hydrochloric acid-water (V / V, 1:100) system, The volume of dialysis is 1000mL, and the dialysis medium is changed every 4 hours for a total of 24 hours of dialysis; finally, part of the wat...
Embodiment 3
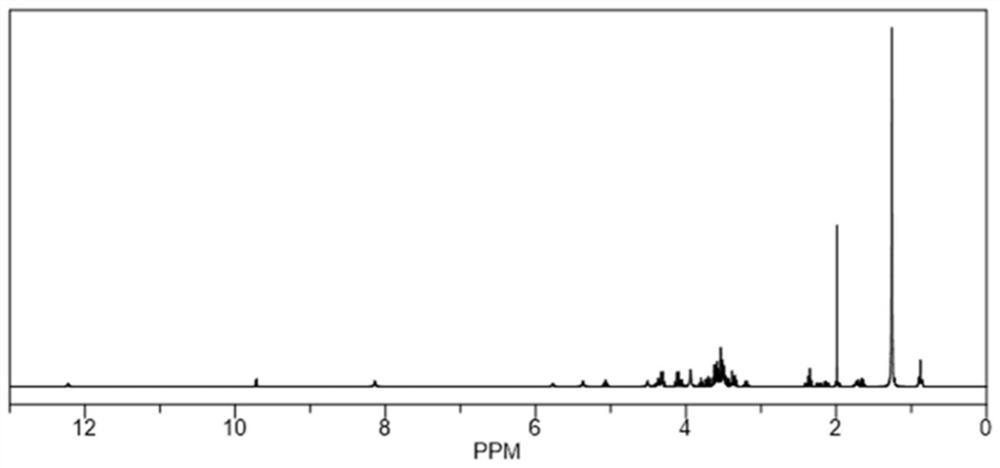
[0053] The synthesis of embodiment 3 polysialic acid-hexapolyglyceryl laurate
[0054] PSA-PG preparation process of the present invention: at first, feed ratio is polysialic acid (PSA) / hexapolyglycerol laurate (PG 6 -C 12 ) / N-hydroxysuccinimide (NHS) / 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) / triethylamine (TEA)=1: 1:2:4:4 (molar ratio); Dissolve an appropriate amount of PSA in 5mL of formamide (FA), add EDC / NHS in proportion, activate at 4°C for 90min, the system is clear at this time; then dissolve in 2mL of FA PG in 6 -C 12 Add to the reaction system, add TEA, stir at room temperature for 48h, the reaction solution is clear; then transfer the reaction solution to a dialysis bag (molecular weight cut-off 10kDa), the dialysis medium is concentrated hydrochloric acid-water (V / V, 1:100) system, The dialysis volume is 1000mL, and the dialysis medium is changed every 4 hours for a total of 24 hours of dialysis; finally, part of the water is removed by ro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com