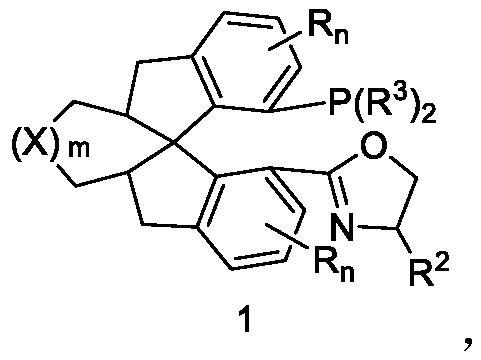
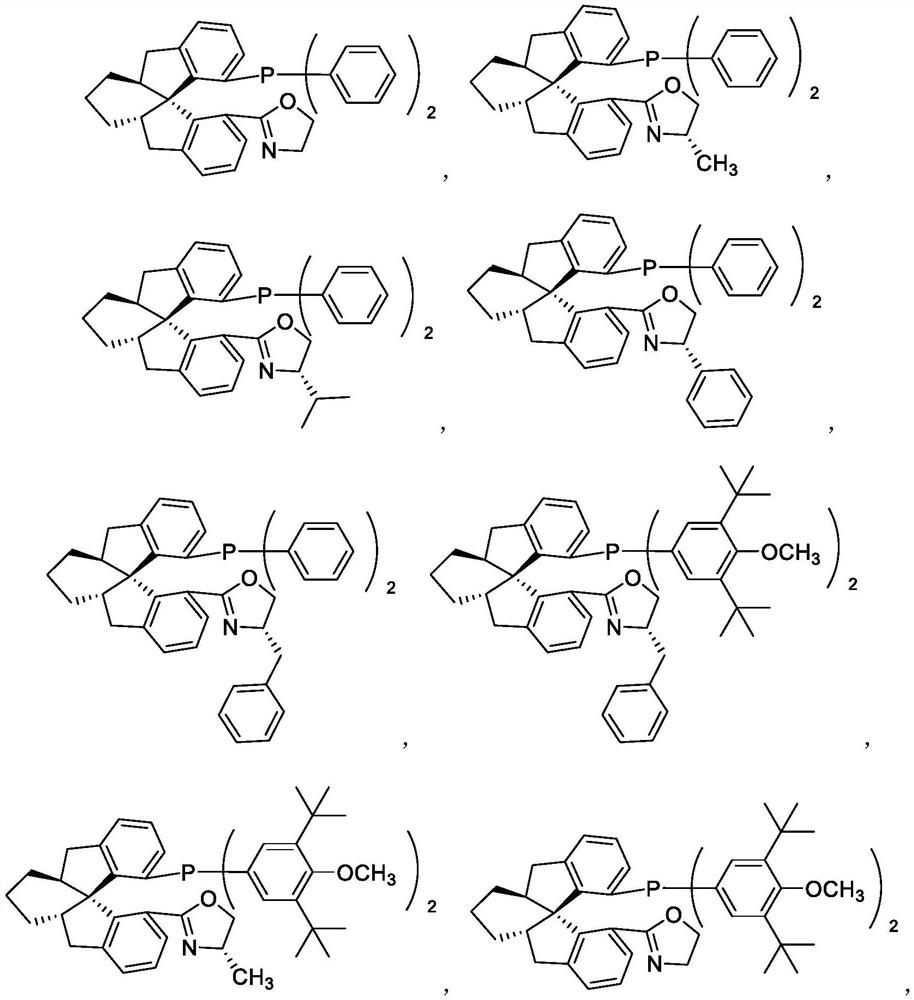
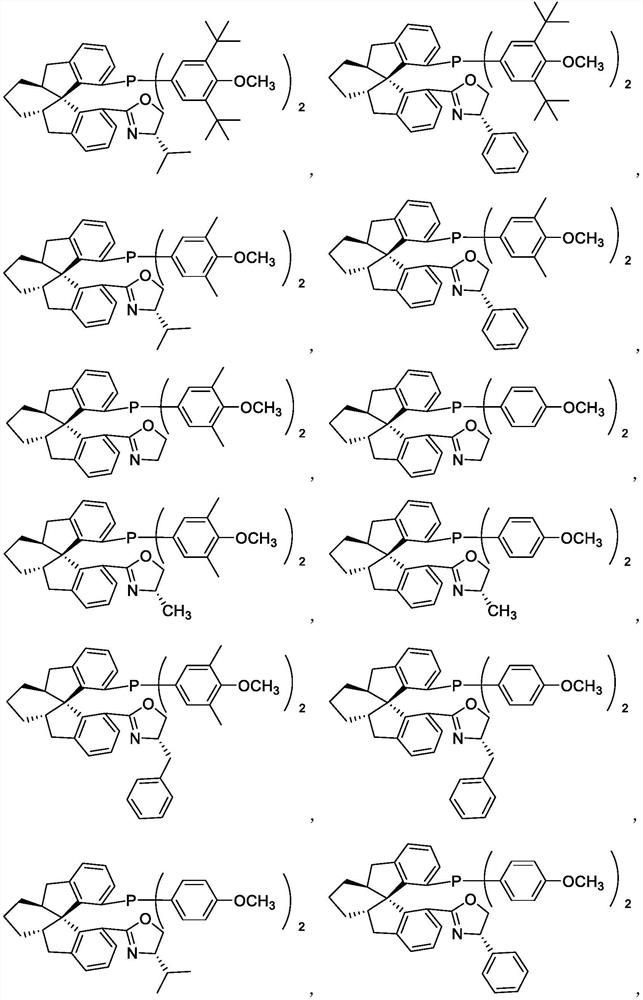
Chiral spiro monophosphine-oxazoline ligands and preparation method thereof
An oxazoline and spiro ring technology, applied in the field of organic synthesis, can solve problems such as few catalyst systems, and achieve the effects of high yield, optical purity, and significant technical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Embodiment 1: the preparation of compound 5
[0225]
[0226] Add (S,S,S)-6 (17.5 g, 60 mmol), pyridine (14.1 mL, 175.0 mmol) and 200 mL of freshly distilled CH to a 500 mL reaction flask 2 Cl 2 , Add trifluoromethanesulfonic anhydride (25.5 mL, 150 mmol) dropwise at 0°C, after the addition is complete, stir at room temperature overnight. Add dilute hydrochloric acid to quench the reaction, adjust the pH of the aqueous phase to 6-7, CH 2 Cl 2 extraction. Organic phase with saturated NaHCO 3 The solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate. After the filtrate was concentrated, 32.0 g of a light yellow solid was obtained by silica gel column chromatography (eluent: n-hexane / ethyl acetate=10 / 1), yield: 96%.
Embodiment 2
[0227] Embodiment 2: compound 3-1 (R 3 =Ph) Preparation
[0228]
[0229] To a 250mL reaction flask was added bistrifluoromethanesulfonate 5 (15.0g, 27.0mmol) of the spiro compound, diphenylphosphine oxide (7.0g, 35.0mmol), palladium acetate (303mg, 1.35mmol), 1, 4-Diphenylphosphinobutane (dppb, 576 mg, 1.35 mmol) and 70 mL of anhydrous DMSO. Diisopropylethylamine (19 mL, 108.0 mmol) was added under stirring, and heated to 100° C. for 6 hours. Cool to room temperature, add EtOAc / water to dilute, filter, the filtrate is desolventized and subjected to silica gel column chromatography (eluent: n-hexane / ethyl acetate=5 / 1) to obtain 15.0 g, yield 91%; light yellow solid.
Embodiment 3
[0230] Embodiment 3: compound 3-1 (R 3 =Xyl) Preparation
[0231]
[0232] Add bistrifluoromethanesulfonate 5 (15.0 g, 27.0 mmol) of the spiro compound, bis(3,5-dimethylphenyl) phosphine oxide (9.0 g, 35.0 mmol), palladium acetate into a 250 mL reaction flask (303 mg, 1.35 mmol), 1,4-diphenylphosphinobutane (dppb, 576 mg, 1.35 mmol), and 70 mL of anhydrous DMSO. Diisopropylethylamine (19 mL, 108.0 mmol) was added under stirring, and heated to 100° C. for 6 hours. Cooled to room temperature, added EtOAc / water to dilute, filtered, and the filtrate was subjected to silica gel column chromatography (eluent: n-hexane / ethyl acetate=5 / 1) to obtain 15.4 g, yield 86%; light yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com