Product for inhibiting viruses and application
A product, virus technology, applied in the field of virus suppression products, can solve problems such as waste, underutilization of natural resources, pollution of the natural environment, etc., and achieve remarkable effects, remarkable application effects, and remarkable suppression effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
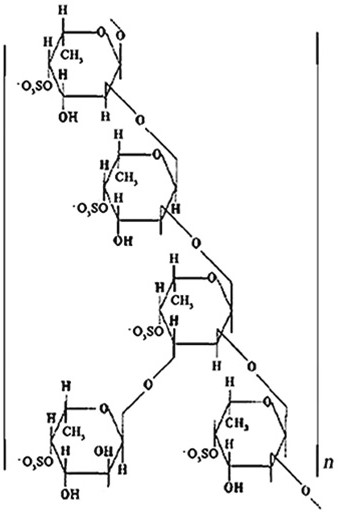
[0035] Example 1 Extraction of Fucoidan Sulfate
[0036] Process 1: The conventional extraction, separation and purification process of laminaria fucoidan sulfate (see the literature "Nishino, T. et al: Carbohydr. Res . 1989, 186:119-129."), its preparation method is as follows:
[0037] Kelp (purchased in the market, 100 grams) → dried (60 o C)→crushing→kelp powder (60 mesh)→add acetone (2,000 mL, soak three times, 12h each time)→filter→filter residue→dry (60 o C) → hot water extraction (water 4 L, soaked three times, 80 ~ 95 o C) → filtrate → concentrated under reduced pressure (65 oBelow C) → ethanol (ethanol concentration reaches 30, 70, 85% respectively) → precipitation → crude fucoidan sulfate → make 3% polysaccharide aqueous solution → anion exchange resin (anion exchange resin of Q- Sepharose FF, use H 2 0, 1.0 mol / L and 2.0 mol / L NaCl solution for stepwise elution)→concentration under reduced pressure (65 o Below C)→freeze drying→Fucoidan sulfate (brown powder);...
Embodiment 2
[0042] Example 2 Comparison of Extraction Processes of Laminaria Fucoidan Sulfate
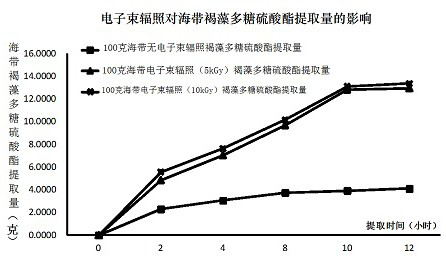
[0043] 1. Experiments were carried out according to the process 1, 2, and 3 for the extraction, separation and purification of fucoidan sulfated laminaria in Example 1, and the weight (mg) of fucoidan laminaria sulfate before and after electron beam irradiation was compared.
[0044] Test results are shown in Tables 1, 2, 3 and figure 1 :
[0045] Extraction time (hours) Experiment 1 Experiment 2 Experiment 3 average Standard deviation (±) 0 0.0000 0.0000 0.0000 0.0000 0.0000 2 2.3010 2.2789 2.3109 2.2969 0.0164 4 3.0123 3.0563 3.0875 3.0520 0.0378 S 3.6801 3.7761 3.7901 3.7488 0.0599 10 3.9806 3.8991 3.9012 3.9270 0.0465 12 4.1205 4.0789 4.0945 4.0980 0.0210
[0046] Promotion time (hours) Real light 1 Experiment 2 Real lights 3 average Standard deviation (±) 0 0.0000 0.0000 0.0000 0.0000...
Embodiment 3
[0064] A virus-inhibiting product, the raw materials and parts by weight are 9 parts of sea salt, 0.1 part of sodium bicarbonate, 0.3 part of citric acid, 0.2 part of sodium citrate, 0.5 part of fucoidan sulfate, prepared into 0.6% by using clean water as a solvent Nasal rinse / nasal spray in (gram / ml) concentration for use in combination with nasal spray or nebuliser for the prevention and treatment of COVID-19 / flu, silicosis, and the treatment of rhinitis, and / or sinusitis, and / or allergic rhinitis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com