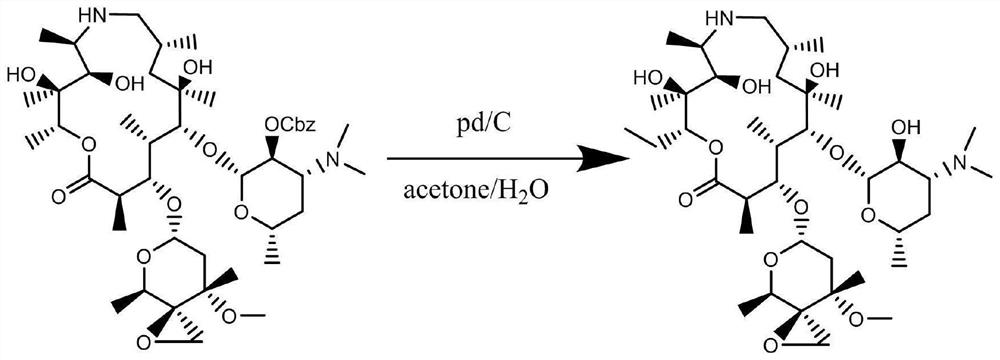
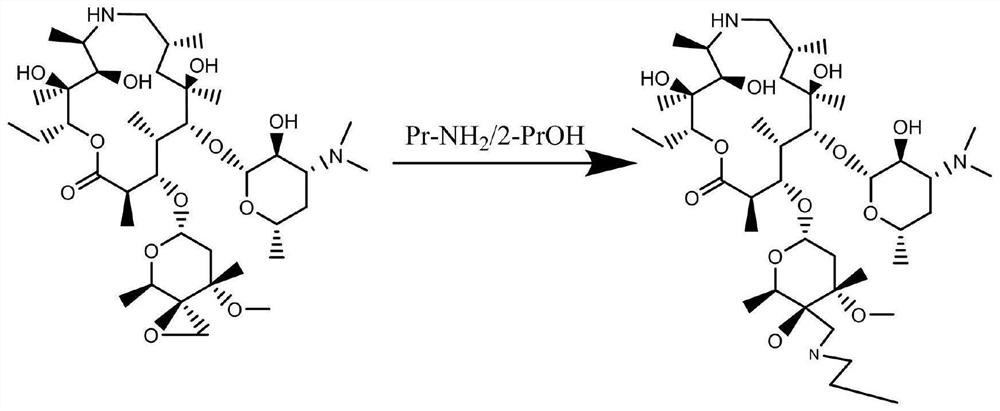
Method for synthesizing and purifying tulathromycin impurity E
A technology of telamycin and impurities, which is applied in the field of medicinal chemistry, can solve the problems of low purity of telamycin impurity E, unsatisfactory structure and properties, and difficult control of the synthesis process, so as to improve purity, promote conversion efficiency, and improve properties. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The method for synthesizing and purifying teramycin impurity E of the present embodiment comprises the following steps:
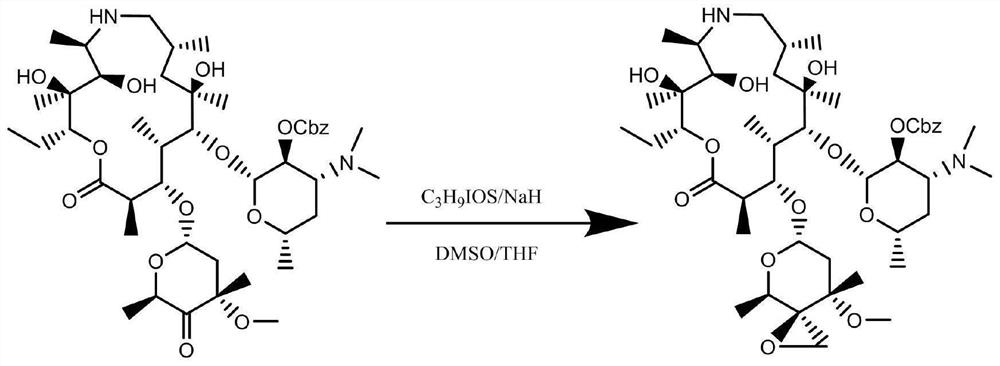
[0042] (1) Add 3.0g of potassium hexamethyldisilazide, 100ml of DMSO and tetrahydrofuran to a 500ml four-necked flask, stir to dissolve, pass N2 for protection, add 15.7g of trimethylsulfoxide iodide in stages, and control the temperature to 0 ℃, keep stirring at this temperature for 2 hours; add DMSO solution of teramycin oxide (30g+100ml) dropwise, control the dropping temperature at 30 ℃, after the dropwise addition, keep the reaction at -50 ℃ for 1 hour, take samples for detection , epoxy idene ketone isomer: epoxy compound = 85:15, the reaction solution was added to 10% ammonium chloride aqueous solution to quench the reaction, the reaction solution was layered, washed with brine, and the organic phase was concentrated to dryness to obtain a ring 30.4 g of crude oxalide isomer;
[0043] (2) Refining the crude epoxy ketone isomer: dissolve the c...
Embodiment 2
[0048] The method for synthesizing and purifying teramycin impurity E of the present embodiment comprises the following steps:
[0049] (1) To a 500ml four-necked flask, add 3.0g of sodium hydride, 100ml of DMSO and tetrahydrofuran in total, stir to dissolve, pass in N2 for protection, add 15.7g of trimethyl sulfoxide iodide in stages, control the temperature to 20°C, and keep the temperature at this temperature. Stir for 2 hours; add DMSO solution of teramycin oxide (30g+100ml) dropwise, control the dropwise temperature at -30°C, keep the temperature at -50°C for 1 hour after the dropwise addition, take samples for detection, epoxy alkenone Isomer: epoxy compound = 90:10, the reaction solution was added to 10% ammonium chloride aqueous solution to quench the reaction, the reaction solution was separated into layers, washed with brine, and the organic phase was concentrated to dryness to obtain epoxy idene ketone isomerization Crude product 31.4g;
[0050] (2) Refining the cr...
Embodiment 3
[0055] The method for synthesizing and purifying teramycin impurity E of the present embodiment comprises the following steps:
[0056] (1) To a 500ml four-necked flask, add 3.0g of sodium hydride, a total of 100ml of DMSO and tetrahydrofuran, stir and dissolve, pass into N2 for protection, add 15.7g of trimethyl sulfoxide iodide in stages, control the temperature to 10°C, and keep the temperature at this temperature. Stir for 2 hours; add DMSO solution of teramycin oxide (30g+100ml) dropwise, control the temperature of dropwise addition at 0°C, and keep the temperature at -50°C for 1 hour after the dropwise addition is completed. Structure: epoxy compound = 90:10, the reaction solution was added to 10% ammonium chloride aqueous solution to quench the reaction, the reaction solution was separated into layers, washed with brine, and the organic phase was concentrated to dryness to obtain epoxyidene ketone isomers Crude product 30.0g;
[0057] (2) Refining the crude epoxy keton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com