Layered double-metal-based nano lanthanum material as well as preparation method and application thereof
A layered bimetallic and bimetallic salt technology is applied in chemical instruments and methods, other chemical processes, water/sludge/sewage treatment, etc. Low problems, to achieve the effect of phosphorus removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1) Prepare 2.25mol L -1 MgCl 2 and 0.75mol L -1 FeCl 3 The mixed solution of bimetallic salt is solution A, and 2mol L of precipitant is prepared -1 NaOH and 0.2mol L -1 Na 2 CO 3 is solution B;
[0047] 2) Slowly pump solution A and solution B into the system at the same time for co-precipitation reaction, and keep stirring to mix the reaction system evenly, during which the reaction pH=10.0 is kept constant at a specific value until the end of the reaction;
[0048] 3) Place the reaction product obtained in step 2 in a water bath at 65°C for 18 hours to crystallize by heating in a water bath;
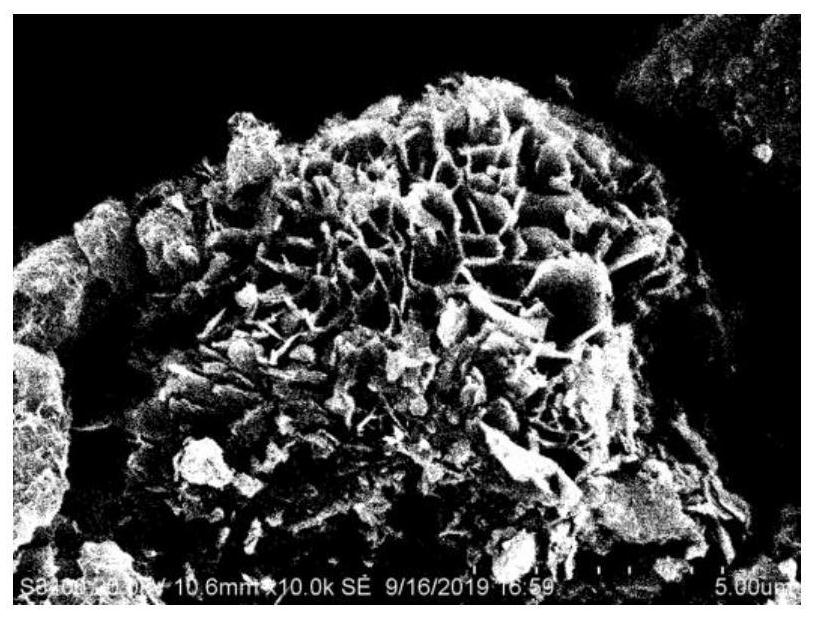
[0049] 4) The product in step 3 is centrifuged, washed with distilled water until neutral, dried, and ground into powder to obtain the product Mg / Fe-LDHs.
[0050] 5) Calcining the above Mg / Fe-LDHs powder material in a muffle furnace at a certain temperature of 400°C for 2 hours to obtain Mg / Fe-LDOs;
[0051] 6) Add Mg / Fe-LDOs to 3.75gLa·L -1 (10g·L -1 LaCl 3 ·7H 2...
Embodiment 2
[0056] 1) Prepare 2.25mol L -1 MgCl 2 and 0.75mol L -1 AlCl 3 The mixed solution of bimetallic salt is solution A, and 2mol L of precipitant is prepared -1 NaOH and 0.2mol L -1 Na 2 CO 3 is solution B;
[0057]2) Slowly pump solution A and solution B into the system at the same time for co-precipitation reaction, and keep stirring to mix the reaction system evenly, during which the reaction pH=9.5 is kept constant at a specific value until the end of the reaction;
[0058] 3) The reaction product obtained in step 2 was placed in a certain 80°C water bath for 12 hours to carry out heating and crystallization in the water bath;
[0059] 4) The product in step 3 is centrifuged, washed with distilled water until neutral, dried, and ground into powder to obtain the product Mg / Al-LDHs.
[0060] 5) Calcining the above Mg / Al-LDHs powder material in a muffle furnace at a certain temperature of 500°C for 2 hours to obtain Mg / Al-LDOs;
[0061] 6) Add Mg / Al-LDOs to 12.5gLa·L -1 ...
Embodiment 3
[0066] Steps 1) to 5) are the same as in Example 1, and the alcohol solution concentration of lanthanum in step 6) is changed to a series of different concentrations, followed by: 0.75gLa L -1 (2.0g·L -1 LaCl 3 ·7H 2 O), 1.875gLa·L -1 (5.0g·L -1 LaCl 3 ·7H 2 O), 3.75gLa·L -1 (10.0g·L -1 LaCl 3 ·7H 2 O), 6.25gLa·L -1 (16.67g·L -1 LaCl 3 ·7H 2 O), the solid-to-liquid ratio was changed to 1:100.
[0067] The material L-CMF-x obtained in this example is in the form of brown powder, and its lanthanum loading measured by ICP after digestion is: 6.79%, 14.70%, 21.9%, 41.34%; the adsorption experiment results show that L-CMF-x PO 4 The adsorption capacity is: 42.64mgP·g -1 , 55.02mgP·g -1 , 64.88mgP·g -1 , 89.70mgP·g -1 . The above results are compared with Example 1 to illustrate that the L-CMF-x series materials with Mg / Fe-LDHs as the carrier can obtain materials with different lanthanum loadings by adjusting the concentration of the alcohol solution of lanthanum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com