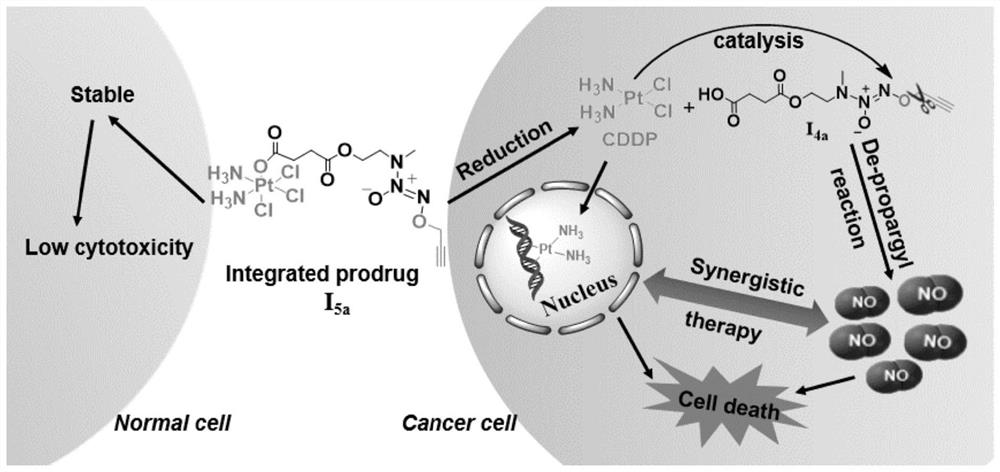
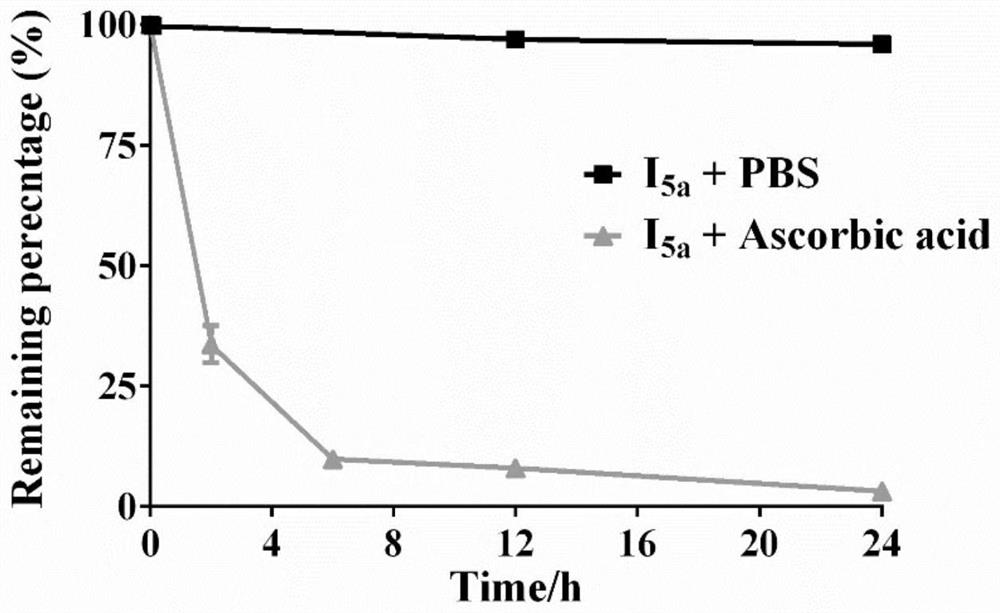
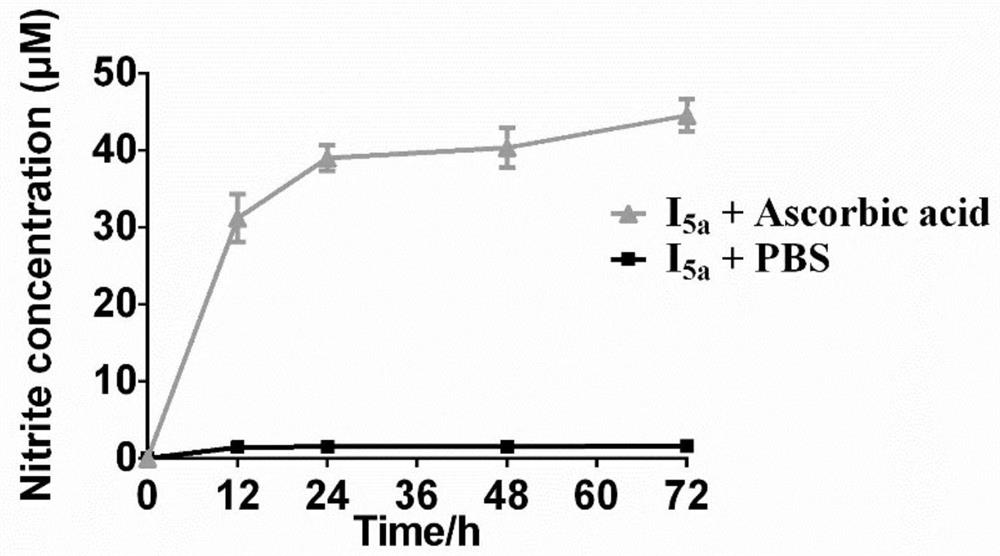
Integrated prodrug based on bioorthogonal chemistry as well as preparation method and medical application of integrated prodrug
A pharmacy and methyl technology, applied in the field of pharmacy, can solve the problems of unclear direct action mechanism and affecting DNA replication of tumor cells, so as to improve druggability, avoid toxic and side effects, and avoid separate drug administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Compound I 5a , I 5b preparation of
[0054]
[0055] synthetic route:
[0056]
[0057]
[0058] CH 3 ONa: sodium methoxide; CH 3 OH: methanol; DMF: N,N-dimethylformamide; THF: tetrahydrofuran; CHP: cis-diamine trichloroplatinum; FHP: trans-diamine trichlorohydrin; Et 3 N: triethylamine; TBTU: 2-(1H-benzotrisazoL-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate.
[0059] Take 217mg of Compound I 4 Add 86.6 mg of triethylamine, 265 mg of TBTU, and 5 mL of DMF into a single-necked reaction flask, and stir the reaction solution at room temperature for 30 mins. Then, 200 mg of CHP or FHP was added to the reaction bottle, and the reaction solution was kept in the dark for overnight. Post-processing, concentrate first to go out DMF, make sand from the residue, and obtain the target product I by column chromatography 5a and I 5b .
[0060] Compound I 5a : Pale yellow solid, 97 mg, yield 28%. 1 H NMR (ppm, 300MHz, DMSO-d 6 )δ6.28-6.03(m, 6H),...
Embodiment 2
[0062] Embodiment 2: Compound I 5c preparation of
[0063]
[0064] synthetic route:
[0065]
[0066]
[0067] CH 3 ONa: sodium methoxide; CH 3 OH: Methanol; CH 3 I: methyl iodide; DMF: N,N-dimethylformamide; THF: tetrahydrofuran; CHP: cis-diamine trichlorohydrin; Et 3 N: triethylamine; TBTU: 2-(1H-benzotrisazoL-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate.
[0068] Take 200mg of Compound I 4 In a single-necked reaction flask, add 86.6 mg of triethylamine, 265 mg of TBTU, and add 5 mL of DMF to dissolve, and the reaction solution is stirred at room temperature for 30 min. Then, 200 mg CHP was added into the reaction bottle, and the reaction solution was continued to react overnight under dark conditions. Post-processing, first concentrated to go out DMF, residue sand making, column chromatography obtains the target product 134mg of I 5c , yield 37%. 1 H NMR (ppm, 500MHz, DMSO-d 6)δ6.28-6.03(m,6H),4.15(t,J=5.0Hz,2H),3.90(s,3H),3.52(t,J=5.0Hz,2H),2.95(s...
Embodiment 3
[0082] Embodiment 3: research compound I 5a Anti-tumor proliferation inhibitory activity in zebrafish.
[0083] First, a certain amount of CellTracker was transplanted into the yolk of juvenile zebrafish TM CM-Dil fluorescent dye-labeled A2780 cells, and then let the zebrafish grow for 24 hours. Under the confocal microscope, it can be seen that the yolk part of the zebrafish has strong yellow fluorescence, so as to construct a zebrafish experimental model of successful tumor cells. Next, the zebrafish that had been implanted with tumor cells were mixed with I 5a (1 μM), cisplatin (1 μM) and zebrafish culture medium without adding other compounds, after 72 hours, observe the yellow fluorescence distribution in the zebrafish body under a confocal microscope, and count the corresponding fluorescence intensity values to evaluate compound I 5a Anti-tumor proliferation inhibitory effect. Figure 10 for further study of compound I 5a Anti-tumor proliferation inhibitory activi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com