Method for continuously preparing (R)-4-halo-3-hydroxy-butyrate by using micro-reaction system
A micro-reaction, butyrate technology, applied in biochemical equipment and methods, carboxylate preparation, chemical instruments and methods, etc., can solve the problems of slowing down the reaction rate, affecting the selectivity of the reaction, and low yield, etc. Achieve the effect of high degree of automation, improved product yield and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
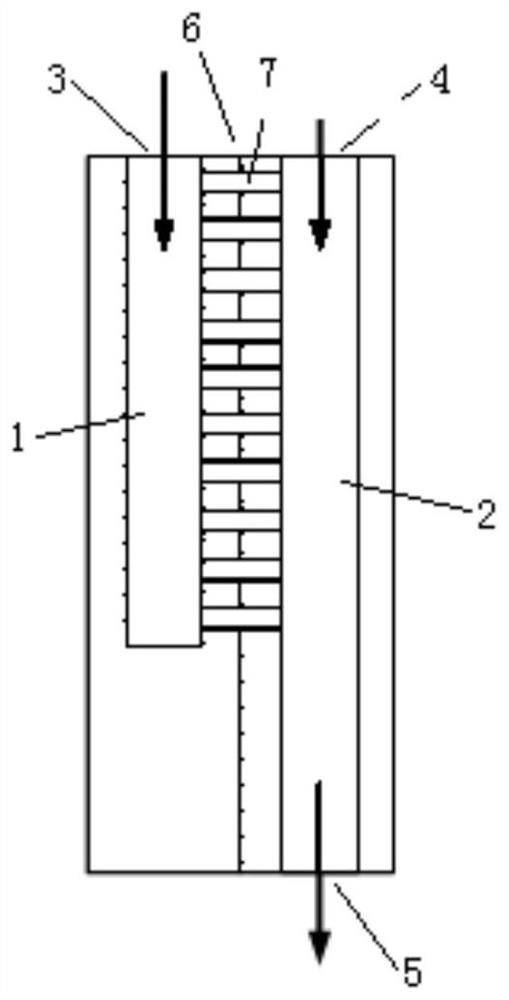
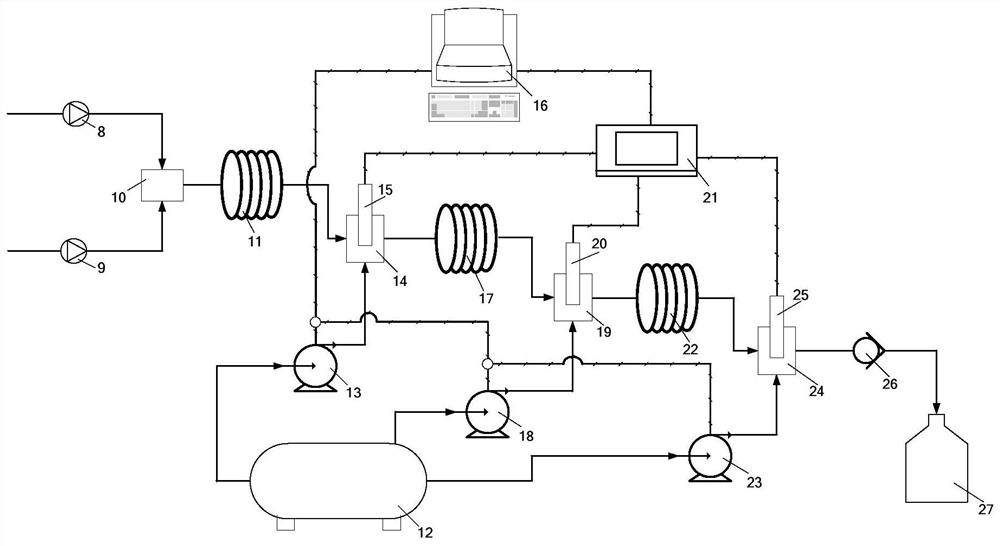
[0070] This embodiment provides a method for continuously preparing (R)-4-chloro-3-hydroxy-butyric acid ethyl ester using a micro-reaction system, such as figure 2 Shown, comprise the micromixer 10 that communicates successively, microchannel reactor 11, pH regulator 14, microchannel reactor 17, pH regulator 19, microchannel reactor 22, pH regulator 24, back pressure valve 26, The product liquid collection storage tank 27 also includes a computer 16, a pH meter body 21, a pH measuring probe 15, a pH measuring probe 20, a pH measuring probe 25, a pH regulator delivery pump 13, a pH regulator delivery pump 18, a pH regulator Delivery pump 23 and pH regulator storage tank 12.
[0071] The pH measuring meter body 21 , the pH measuring probe 15 , the pH measuring probe 20 and the pH measuring probe 25 form a pH measuring meter.
[0072] The computer 16, the pH meter body 21, the pH measuring probe 15, the pH measuring probe 20, the pH measuring probe 25, the pH regulator delivery...
Embodiment 2
[0085] The micro-reaction system used in this embodiment, the experimental operation steps, methods and conditions are all the same as in Example 1, the only difference is that the pH value of the reaction mixture in the pH regulator 14, the pH regulator 19 and the pH regulator 24 is determined by The pH automatic adjustment system is stably controlled at 7.0.
[0086] Sampling and analysis were performed by Agilent liquid chromatography for quantitative detection, and the concentration of reaction substrate and product was quantified by peak area. After analysis, the reaction substrate ethyl chloroacetoacetate was completely converted, and the yield of the product (R)-4-chloro-3-hydroxybutyric acid ethyl ester was 98.7%.
Embodiment 3
[0088] The micro-reaction system used in this embodiment, the experimental operation steps, methods and conditions are all the same as in Example 1, the only difference is that the pH value of the reaction mixture in the pH regulator 14, the pH regulator 19 and the pH regulator 24 is determined by The pH automatic adjustment system is stably controlled at 6.2.
[0089] Sampling and analysis were performed by Agilent liquid chromatography for quantitative detection, and the concentration of reaction substrate and product was quantified by peak area. After analysis, the reaction substrate ethyl chloroacetoacetate was completely converted, and the yield of the product (R)-4-chloro-3-hydroxybutyric acid ethyl ester was 95.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com