Class of fused xylanase mutants with high specific activity and application thereof
A xylanase mutation and xylanase technology, which is applied in the field of functional gene modification, can solve the problems of poor stability of fusion proteins, easy to contaminate bacteria, and can not fully meet the needs of industrialization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The construction of embodiment 1 fusion xylanase mutant engineering bacteria
[0027] In this study, by comparing the amino acid sequences of the same family proteins, the thermal stability-related amino acid sites of the fusion xylanase (Chinese patent document CN108570107A) were predicted by using the FireProt online tool and the principle of sequence consistency, and the following mutation hotspots Y224, W225 were obtained, Q226, G232, V238, S241, N251, S398, use the Accessible Surface Area and Accessibility Calculation for Protein (ver.1.2) online server to calculate the amino acid residues before and after the mutation, and build a library according to the relative distance between the mutation site and the active center (library 1-Y224 / Q226 / N251, Bank 2-W225, Bank 3-G232 / S241, Bank 4-S398). The single point mutation primers used are as follows:
[0028] Primer Sequence(5'to 3') Y224X-F AAAGCTAGCACAGACnnnTGGCAAAATTGGACT SEQ ID NO: 3 Y22...
Embodiment 2
[0044] Fermentation of embodiment 2 recombinant xylanase mutants in Escherichia coli
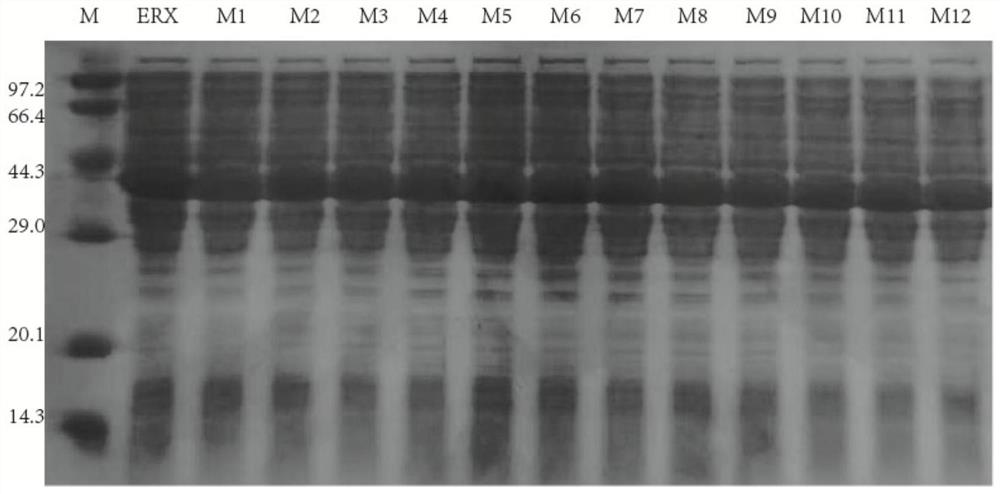
[0045]The 12 combined mutants constructed in Example 1 and the original enzyme ERX were respectively inoculated into 50 mL of LB liquid medium containing 100 μg / mL kanamycin sulfate, cultivated overnight at 37° C. at 180 rpm; Seed solution, inoculated into fresh 50mL LB liquid medium, cultivated to OD at 37°C, 180rpm 600 When it is 0.6-1.0, take it out and cool it in an ice-water bath for 5 minutes, add the inducer IPTG (isopropyl-β-D thiogalactopyranoside) (final concentration 0.1mmol / L), induce expression at 20°C, 150rpm for 20h.
[0046] Get the fermented liquid of induced expression, centrifuge at 12000rpm for 20min, discard the supernatant, then wash with 50mM Na 2 HPO 4 -KH 2 PO 4 (pH 7.0) buffer to resuspend and wash the bacterial cells, centrifuge at 12000rpm for 20min, discard the supernatant, resuspend with buffer, and then sonicate. The broken liquid was centrifuged at 12000r...
Embodiment 3
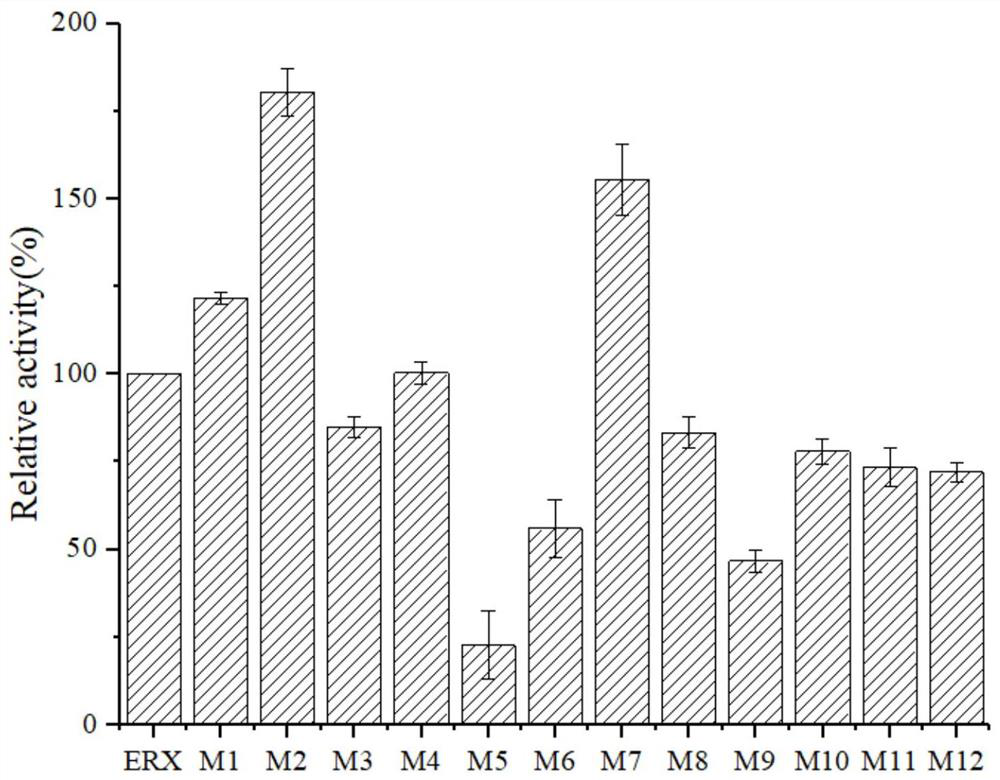
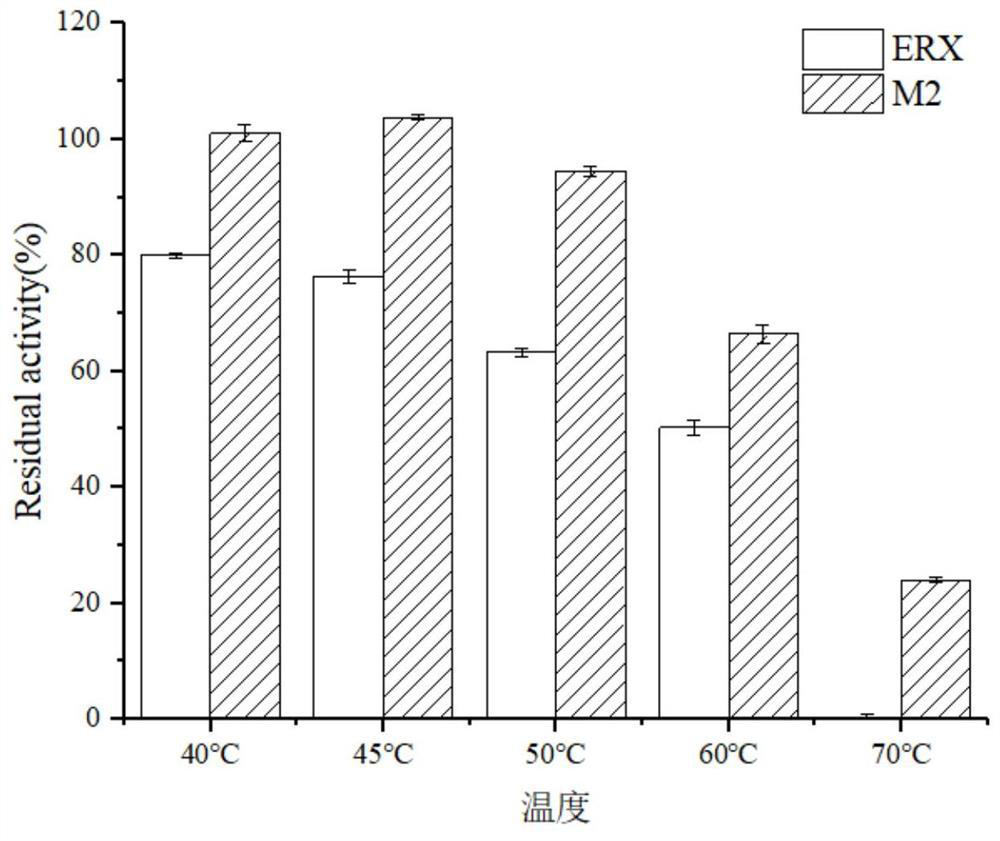
[0048] The screening procedure of embodiment 3 enzyme mutants
[0049] The bacterial strain with the mutated gene constructed in Example 1 was fermented and cultivated according to the method in Example 2, and the obtained fusion protein and its mutant crude enzyme liquid were measured with beech wood xylan as a substrate for enzyme activity changes, and the assay method was as follows:
[0050] Definition of enzyme activity unit: One enzyme activity unit is defined as the amount of enzyme required to produce 1 mmol of reducing sugar from the substrate per minute under the conditions of 60°C and pH 7.0.
[0051] Xylanase (Endo-glucanase): Accurately weigh 1g of beech wood xylan dissolved in 100mL of Na 2 HPO 4 -KH 2 PO 4 Buffer (50mM, pH 7.0), stir and mix, accurately pipette 1.0mL into the test tube as the enzyme reaction substrate, preheat at 60°C for 5 minutes, add 0.5mL of protease solution that has been properly diluted, and put it in a water bath shaker at 60°C for re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com