Preparation method and application of hollow mesoporous inorganic oxide nanosphere solid alkali
A technology of inorganic oxides and hollow mesopores, applied in the preparation of organic compounds, preparation of oxides/hydroxides, preparation/treatment of rare earth metal compounds, etc., to achieve simple preparation process, easy recycling, and increase mass transfer rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation method and application of hollow mesoporous inorganic oxide nanosphere solid base
[0042] (1) Preparation method of hollow mesoporous inorganic oxide nanosphere solid base
[0043] This embodiment includes the following steps:
[0044] S1. Using 1240 g glucose as the carbon source, prepare a 0.5 M glucose solution with deionized water, place the high-pressure hydrothermal reactor in an electric constant temperature drying oven preheated to 200 °C in advance, and then add the glucose solution to the high-pressure water After reacting for 4 hours in a hot reactor, cool it down to room temperature naturally, filter the dark brown product with suction, and wash the filter cake alternately with deionized water and ethanol until the drop at the lower end of the funnel is colorless, then put it in a constant temperature drying oven and dry it at 65 °C for 12 hours , to obtain 55g nano colloidal carbon spheres;
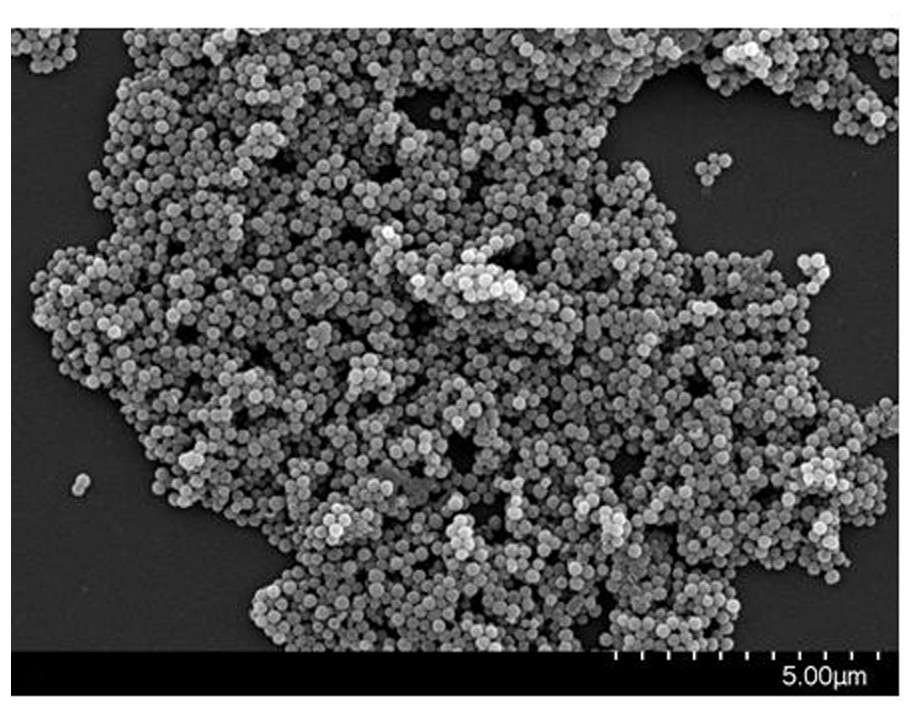
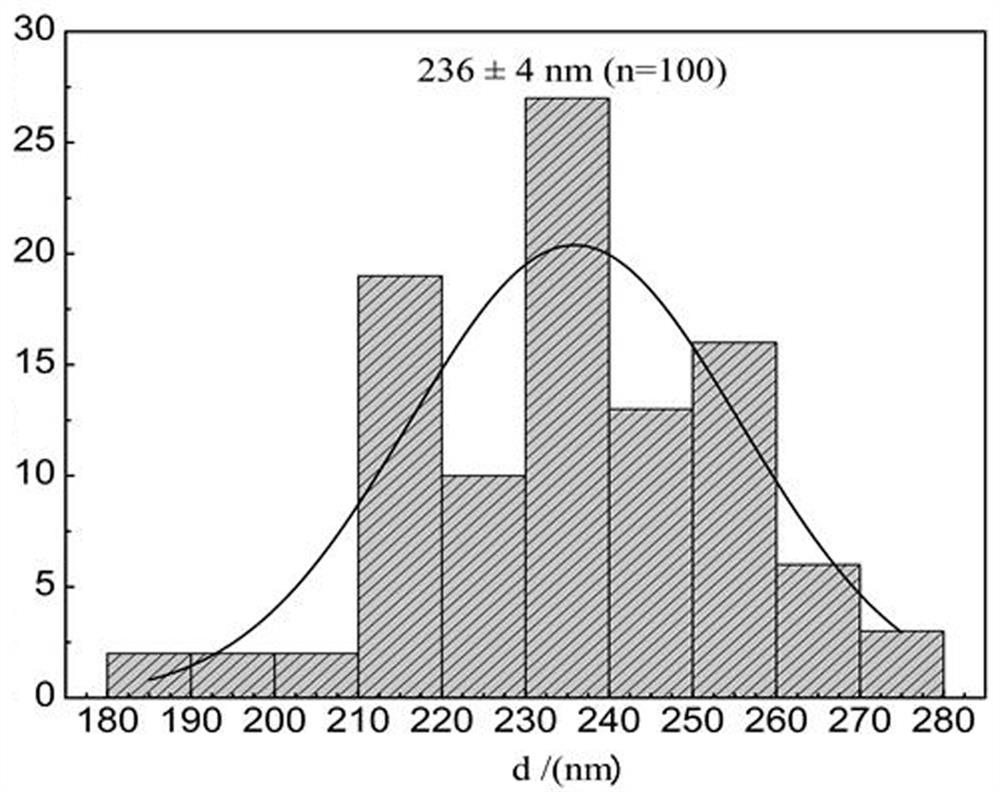
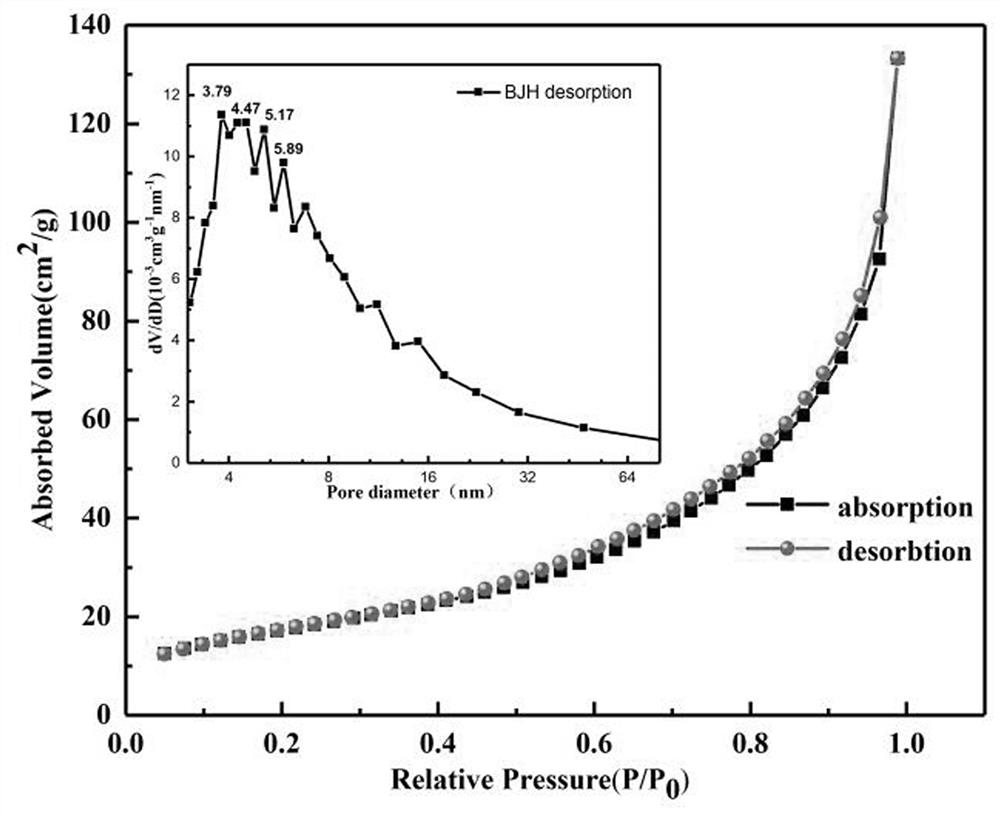
[0045] Such as figure 1 and figure 2 ...
Embodiment 2
[0056] Example 2 Preparation method and application of hollow mesoporous inorganic oxide nanosphere solid base
[0057] (1) Preparation method of hollow mesoporous inorganic oxide nanosphere solid base
[0058] This embodiment includes the following steps:
[0059] S1. Using 1462g of glucose as the carbon source, prepare a 0.8 M glucose solution with deionized water, place the high-pressure hydrothermal reactor in an electric constant temperature drying oven preheated to 160 °C in advance, and then add the glucose solution to the high-pressure hydrothermal After reacting in the reactor for 2 h, cool down to room temperature naturally, filter the dark brown product with suction, and wash the filter cake alternately with deionized water and ethanol until the drop at the lower end of the funnel is colorless, then put it in a constant temperature drying oven at 60 °C for 12 h, Obtain 100g nano colloidal carbon spheres;
[0060] S2. Dissolve magnesium acetate and zinc acetate in ...
Embodiment 3
[0068] Example 3 Preparation method and application of hollow mesoporous inorganic oxide nanosphere solid base
[0069] (1) Preparation method of hollow mesoporous inorganic oxide nanosphere solid base
[0070] This embodiment includes the following steps:
[0071] S1. Using 78g of sucrose as the carbon source, prepare a 1.5 M sucrose solution with deionized water, place the high-pressure hydrothermal reaction kettle in an electric heating constant temperature drying oven preheated to 175 ℃ in advance, and then add the sucrose solution to the high-pressure hydrothermal After reacting in the reactor for 8 hours, it was naturally cooled to room temperature, and the dark brown product was suction-filtered, and the filter cake was washed alternately with deionized water and ethanol until the drop at the lower end of the funnel was colorless, and then dried in a constant temperature drying oven at 70 °C for 12 hours to obtain 20g nano colloidal carbon spheres;
[0072] S2. Magnes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com