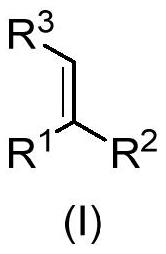
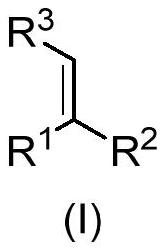
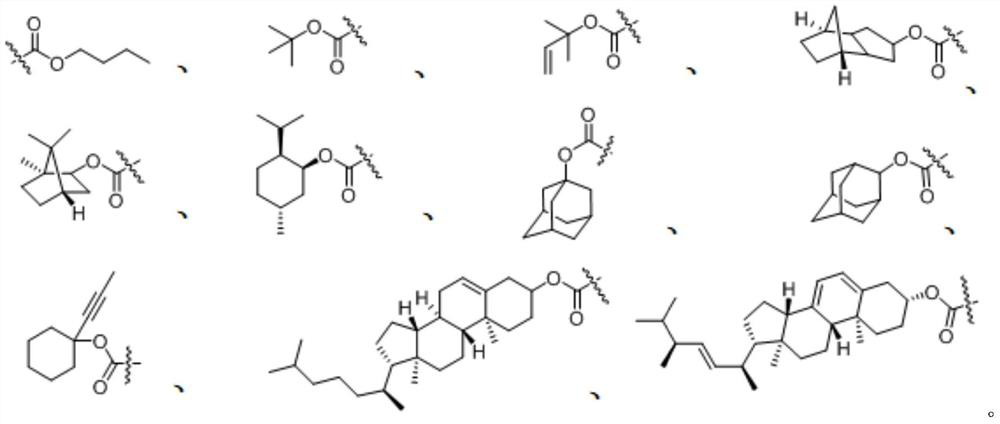
Gamma-amino acid analogue and synthesis method thereof
A synthesis method, amino acid technology, applied in peptide preparation methods, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of low atom economy and large-scale reactions, and achieve wide selectivity of reaction substrates and cheap raw materials Easy to obtain and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A preferred embodiment of the present invention provides a method for synthesizing γ-amino acid analogues, the specific steps are as follows:
[0041]
[0042] S1: To a dry Schlenk tube (10 mL), add 1 (0.3 mmol, 1.0 equiv), Ir[dF(CF 3 )(ppy)] 2 (dtbbpy)·PF 6 (3.3mg, 0.003mmol, 1mol%), 2a (97.3mg, 0.39mmol, 1.3eq)
[0043] S2: add Cs again 2 CO 3 (147.0mg, 0.45mmol, 1.5 equiv)
[0044] S3: Then vacuumize and replace nitrogen for 3 times. Then in N 2 DMF (2.5 mL) was added under atmosphere.
[0045] S4: Seal the Schlenk tube, stir the reaction in a water bath, and irradiate it with a 30W blue LED light (3cm away, with a cooling fan to keep the reaction temperature at 25-30°C) for 8 hours.
[0046] S5: After the reaction, use 1ml 2N HCl (aq.), 2ml H 2 O was quenched, diluted with 3ml EtOAc, stirred for 10 min, extracted 5 times with EtOAc, and the combined organic phase was concentrated in vacuo.
[0047] S6: Finally, the product is purified by silica gel colu...
Embodiment 2
[0049] A preferred embodiment of the present invention provides a method for synthesizing γ-amino acid analogues, the specific steps are as follows:
[0050]
[0051] S1: To a dry Schlenk tube (10 mL), add 1a (0.3 mmol, 1.0 equiv), Ir[dF(CF 3 )(ppy)] 2 (dtbbpy)·PF 6 (1.7mg, 0.0015mmol, 0.5mol%), 2 (0.45mmol, 1.5eq)
[0052] S2: add CsF (91.2mg, 0.6mmol, 2.0 equiv)
[0053] S3: Then vacuumize and replace nitrogen for 3 times. Then in N 2 DMA (3 mL) was added under atmosphere.
[0054] S4: Seal the Schlenk tube, stir the reaction in a water bath, and irradiate it with a 30W blue LED light (3cm away, with a cooling fan to keep the reaction temperature at 25-30°C) for 8 hours.
[0055] S5: After the reaction, use 1ml 2N HCl (aq.), 2ml H 2 O was quenched, diluted with 3ml EtOAc, stirred for 10 min, extracted 5 times with EtOAc, and the combined organic phase was concentrated in vacuo.
[0056] S6: Finally, the product is purified by silica gel column chromatography (petr...
Embodiment 3
[0058] A preferred embodiment of the present invention provides a method for synthesizing γ-amino acid analogues, the specific steps are as follows:
[0059]
[0060] S1: To a dry Schlenk tube (10 mL), add 1a (0.3 mmol, 1.0 equiv), Ir[dF(CF 3 )(ppy)] 2 (dtbbpy)·PF 6 (1.7 mg, 0.0015 mmol, 0.5 mol%), 2a (0.45 mmol, 1.5 equiv).
[0061] S2: add CsF (137mg, 0.9mmol, 3.0eq); add molecular sieves (200mg).
[0062] S3: Then vacuumize and replace nitrogen for 3 times. Then in N 2 DMA (3 mL) was added under atmosphere.
[0063] S4: Seal the Schlenk tube, stir the reaction in a water bath, and irradiate it with a 30W blue LED light (3cm away, with a cooling fan to keep the reaction temperature at 25-30°C) for 8 hours.
[0064] S5: After the reaction, use 1ml 2N HCl (aq.), 2ml H 2 O was quenched, diluted with 3ml EtOAc, stirred for 10 min, extracted 5 times with EtOAc, and the combined organic phase was concentrated in vacuo.
[0065] S6: Finally, the product is purified by si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com