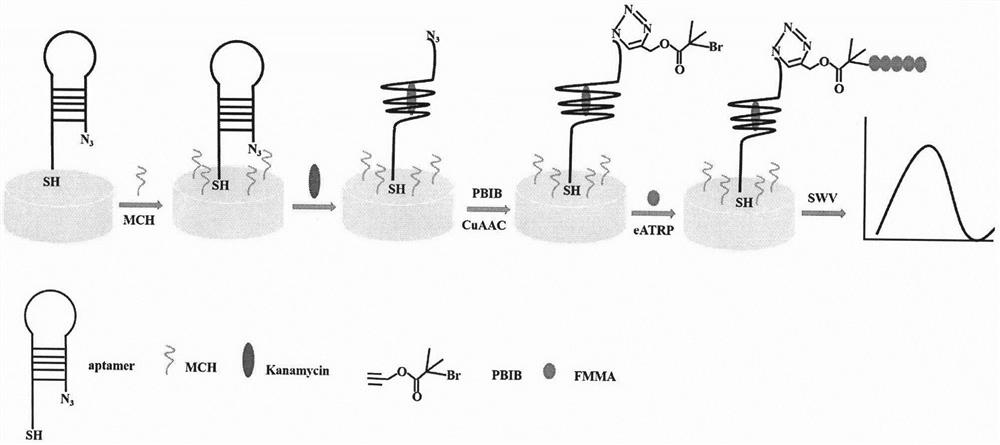
Kanamycin electrochemical detection method based on azido alkyne cycloaddition and electrochemical regulation of atom transfer radical polymerization
A kanamycin and atom transfer technology, applied in the field of life science analysis, can solve the problems of expensive instruments, poor specificity, and complicated detection procedures, and achieve the effects of improved sensitivity, high stability and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1. Feasibility verification
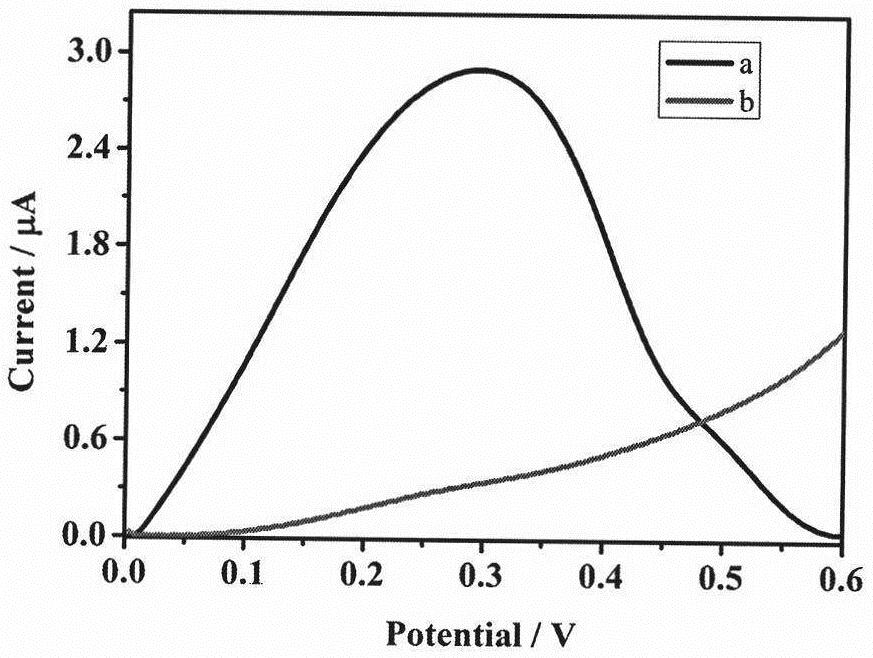
[0025] Firstly, a detection system is constructed on the electrode surface, such as figure 1 shown. Immediately afterwards, the feasibility verification of the detection system is carried out, and the experimental steps are the same as the content of the invention (1)-(6). Such as figure 2 As shown, firstly, without kanamycin (Kana), the hairpin DNA / MCH / PBIB / FMMA modified electrode (curve b) has a weaker current intensity. On the contrary, after adding Kana, the hairpin DNA / MCH / Kana / PBIB / FMMA modified electrode (curve a) can observe an obvious current peak at 0.3 V, which is because a large amount of FMMA was introduced to the gold electrode through eATRP, resulting in a very large current signal. Through comparative experiments, the feasibility of the electrochemical detection method of kanamycin based on azide-alkyne cycloaddition and electrochemical regulation of atom transfer radical polymerization was fully proved.
Embodiment 2
[0026] Embodiment 2. Sensitivity experiment
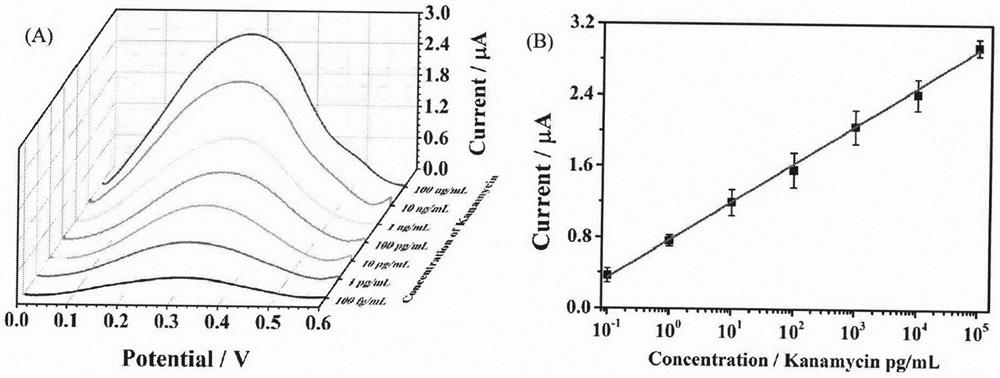
[0027] The experimental procedure is the same as the content of the invention (1)-(6), respectively detecting different concentrations of kanamycin (100fg / mL, 1pg / mL, 10pg / mL, 100pg / mL, 1ng / mL, 10ng / mL, 100ng / mL) to study the detection performance of the inventive method for kanamycin. Such as image 3 As shown, the current intensity increases with the concentration of kanamycin, because the higher the concentration of kanamycin, the farther away from the electrode and the exposed N 3 The higher the number, the more CuAAC is carried out and the more initiator PBIB is introduced, which in turn leads to more FMMA electrochemical polymer monomers and stronger current intensity. The logarithmic value of the maximum peak intensity of the current and the concentration of kanamycin presents a good linear relationship in the range of 100fg / mL to 100ng / mL, and the linear equation is I=0.7716+0.4280logC(R 2 =0.9989), the detection limit w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com