Method for quantitatively determining abiraterone in blood and application thereof
A technology for quantitative determination of abiraterone, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of high cost, high toxicity, and long treatment process, and achieve the use of less sample volume, no matrix interference, and low detection cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
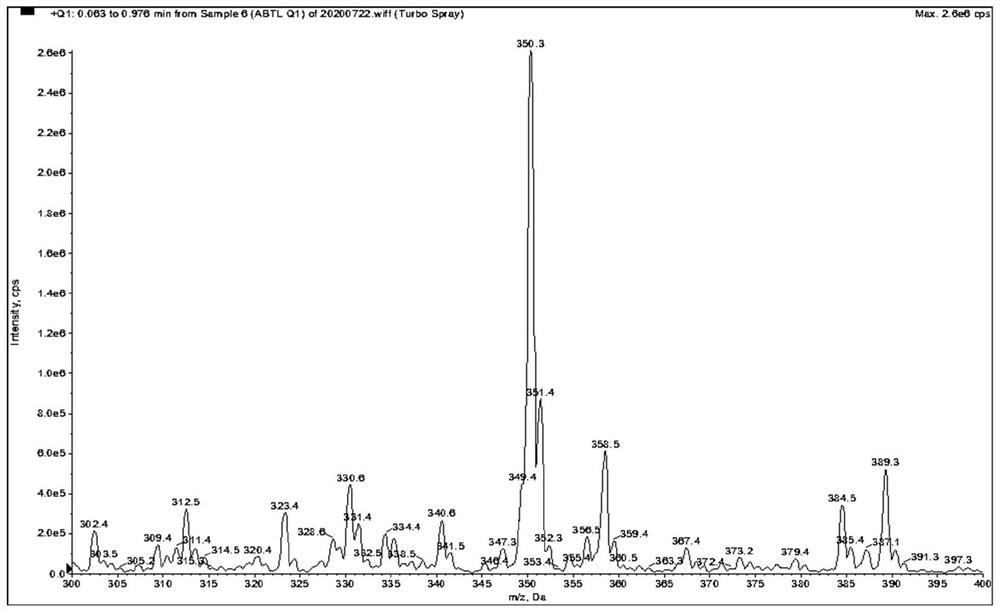
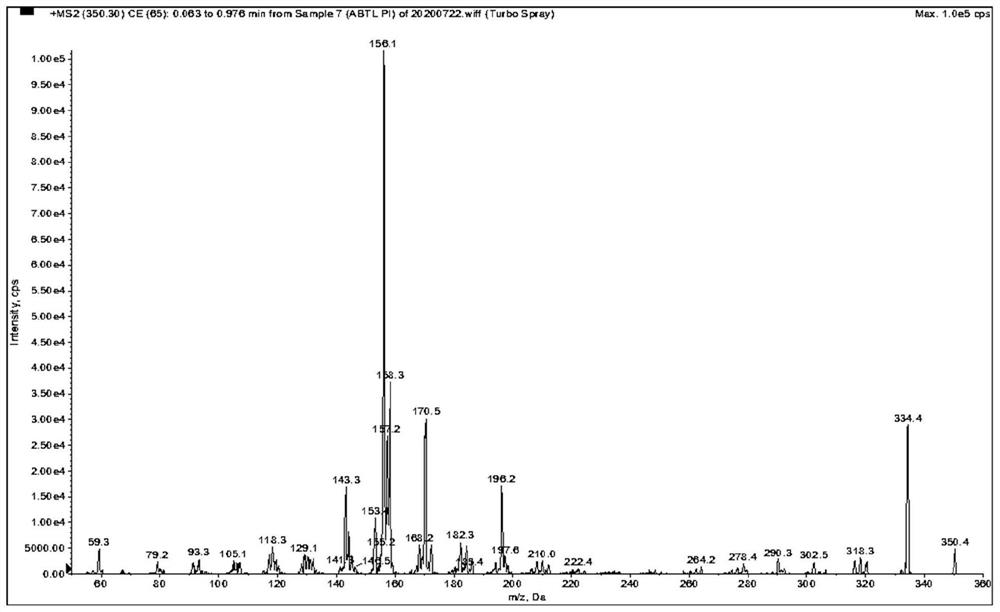
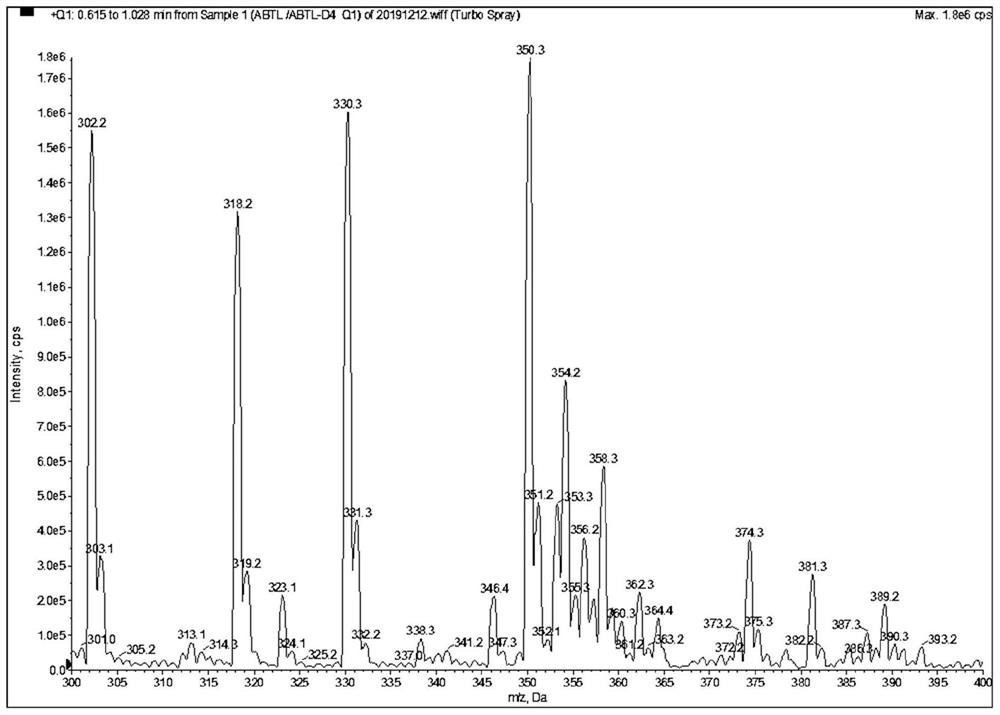
[0078] as attached Figure 1-9 Shown, a method for quantitative determination of abiraterone in blood, specifically comprising the following steps:
[0079] S1: Sample pretreatment
[0080] Take 20.0 μL of plasma / serum sample, add 200 μL of internal standard working solution (acetonitrile solution containing 10 ng / mL Abiraterone D4), vortex for 5 min, then centrifuge at 4°C and 4000 rpm for 10 min, take 180 μL of supernatant, and inject To the liquid mass spectrometer for measurement and analysis.
[0081] S2: Setting parameters
[0082] 1) Set the chromatographic conditions, and the elution parameters are as shown in Table 1.
[0083] Table 1
[0084] time (min) Flow rate (mL / min) Mobile phase A% Mobile phase B% 0.00 0.50 40 60 2.50 0.50 40 60
[0085] Chromatographic column: Agilent ZORBAX Eclipse Plus-C18 chromatographic column, the specification is 2.1×50mm×5μm;
[0086] Guard column: Phenomenex Security Guard Cartridges Kit C18 guar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com