Monoclonal antibody of actinobacillus pleuropneumoniae rApxIVAN and application thereof
A technology of pleuropneumonia and actinobacillus, applied in the field of clinical immunology, can solve problems such as poor sensitivity and achieve the effect of improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
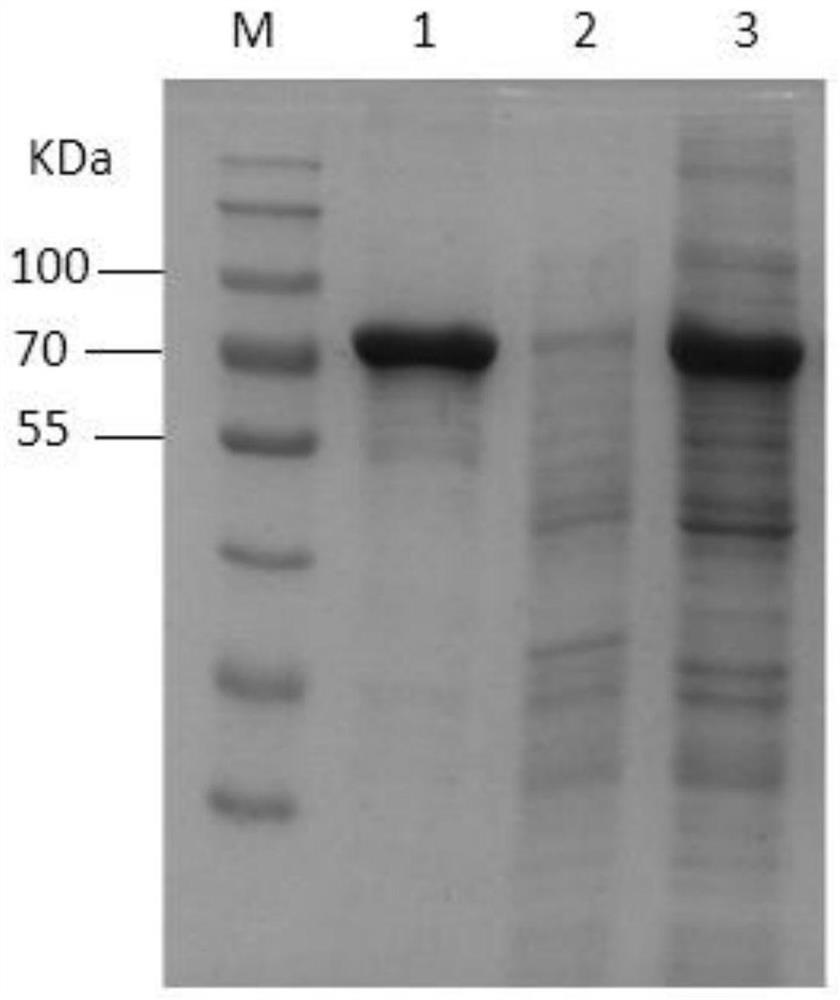
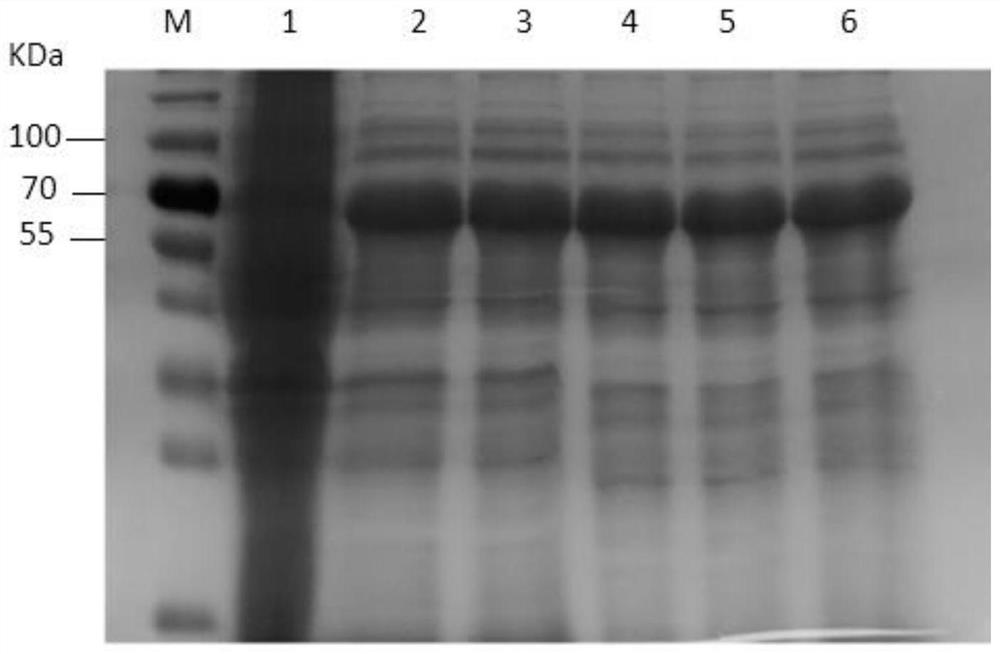
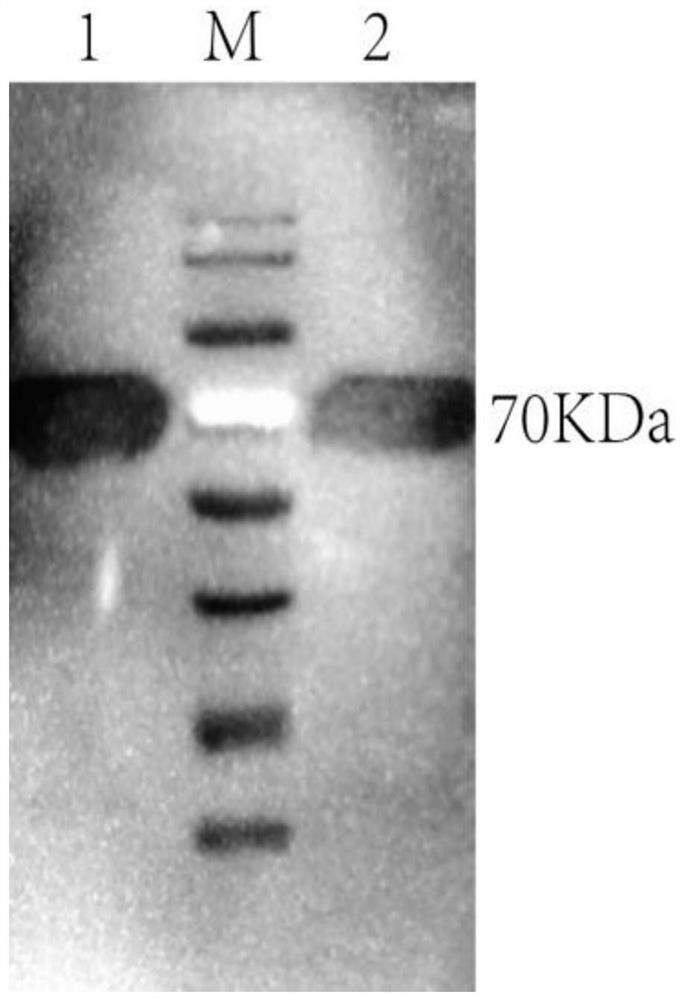
[0025] Obtaining the hybridoma cell line secreting Actinobacillus pleuropneumoniae toxin ApxIVAN monoclonal antibody:
[0026] (1) Transformation of the ApxIVAN antigen gene and protein expression and purification: The APP exotoxin apxIVAN gene was cloned, analyzed and subjected to point mutations, and a region with no signal peptide and rich epitopes was selected for recombinant expression in Escherichia coli. The recombinant plasmid pET20a-apxⅣAN of this sequence induces and expresses the target protein amount accounting for 32.6% of the total bacterial protein. The specific steps are as follows:
[0027] Amplification and transformation of the target gene: using the genomic DNA of Actinobacillus pleuropneumoniae serotype 1 strain as a template, two primers were designed for the apxⅣAN gene (GenBank accession number CP001091), and the upstream primer P1: ATATA CATATG CGCGCCTATATCTGGAATACC, NdeI restriction site; downstream primer P2: ATTG CTCGAG CCCTTCGAATTGTTTCGCATTAAC, ...
Embodiment 2
[0066] Application of a monoclonal antibody against Actinobacillus pleuropneumoniae rApxIVAN in the preparation of an indirect competition ELISA detection kit:
[0067] ① Selection of optimal antigen coating concentration and working concentration of enzyme-labeled monoclonal antibody
[0068]Antigen protein rApxIVAN (initial concentration 162.88 μg / mL) was diluted 6 gradients, coated with ELISA plate, overnight at 4°C; washed 3 times with PBST, blocked with 1% BSA for 2 hours; washed 3 times with PBST, added The 2-fold diluted standard negative serum and positive serum were reacted at 37°C for 1 hour; the plate was washed 3 times with PBST, and the HRP enzyme-labeled monoclonal antibody diluted 1:2000-1:25600 times was added, and the indirect competition ELISA square array test was performed, and the calculation Inhibition rate (Percentage inhibition, PI), PI=(1-positive serum OD 450 nm value / negative serum OD 450 nm value) × 100%, the antigen coating concentration and enzy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com