Niobium tungstate material for high-safety lithium ion battery as well as preparation method and application of niobium tungstate material
A lithium-ion battery, high-safety technology, applied in battery electrodes, secondary batteries, chemical instruments and methods, etc., can solve the problems of low output of electrospinning, inability to mass production, and high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 1. Preparation: hydrate niobium oxalate, ammonium metatungstate and glycine in a molar ratio (n niobium + n tungsten): n glycine = 0.5, wherein, Nb 2 o 5 : WO 3 Molar ratio=7:3, add 7.5molL -1 in dilute nitric acid and stirred to form a clear solution. Set the temperature of the muffle furnace to 1100°C, put the above reaction solution in a corundum crucible with a cover and put it in the muffle furnace. The solution boils and evaporates rapidly, and generates a flame to undergo a self-propagating combustion reaction. The combustion reaction can be completed within 1 minute, and calcined at 1100 ° C for 30 minutes to increase the crystallinity of the product to obtain Nb 14 W 3 o 44 Material.
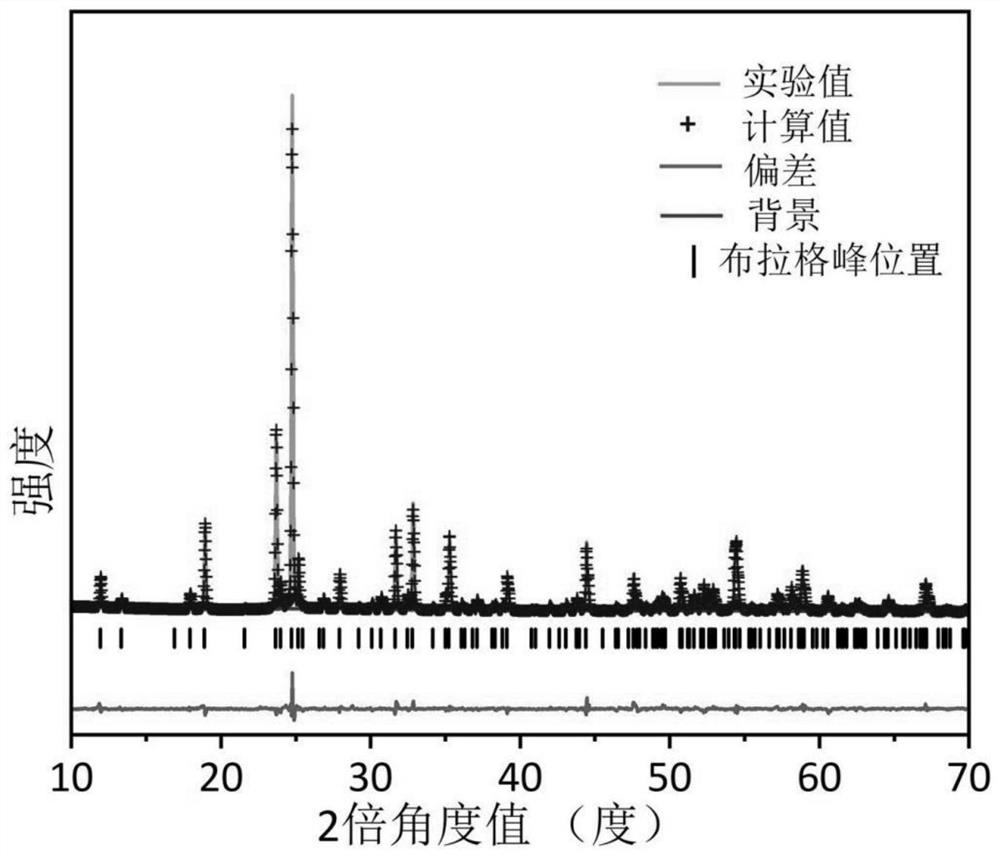
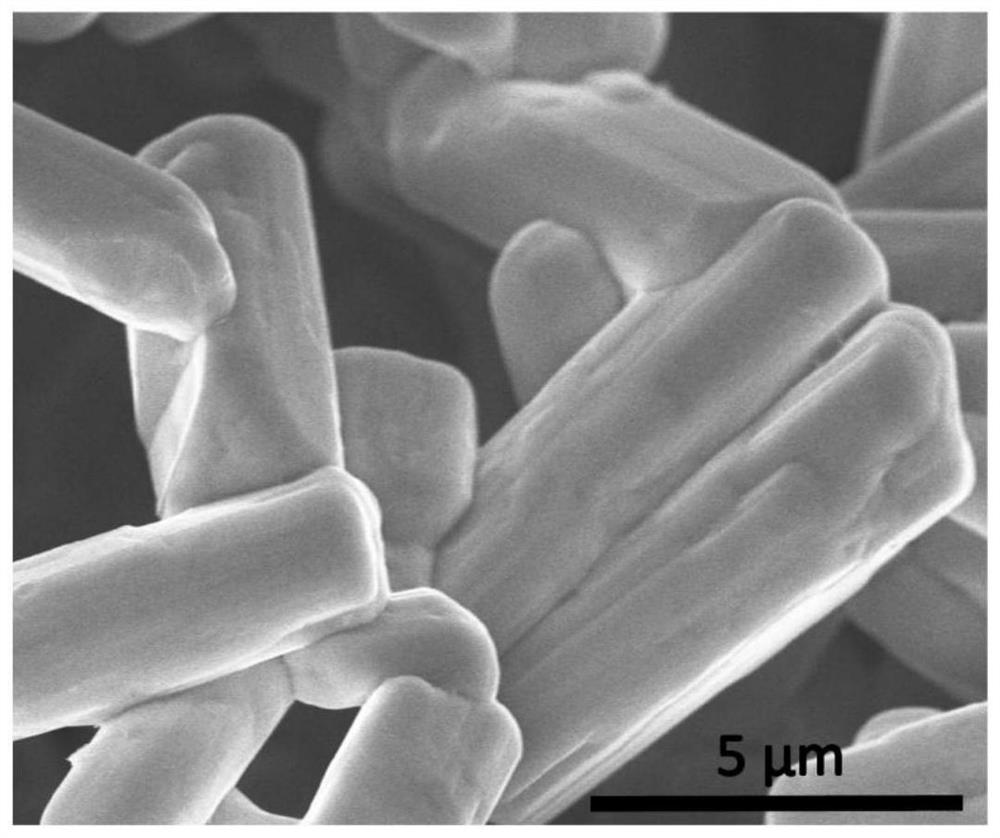
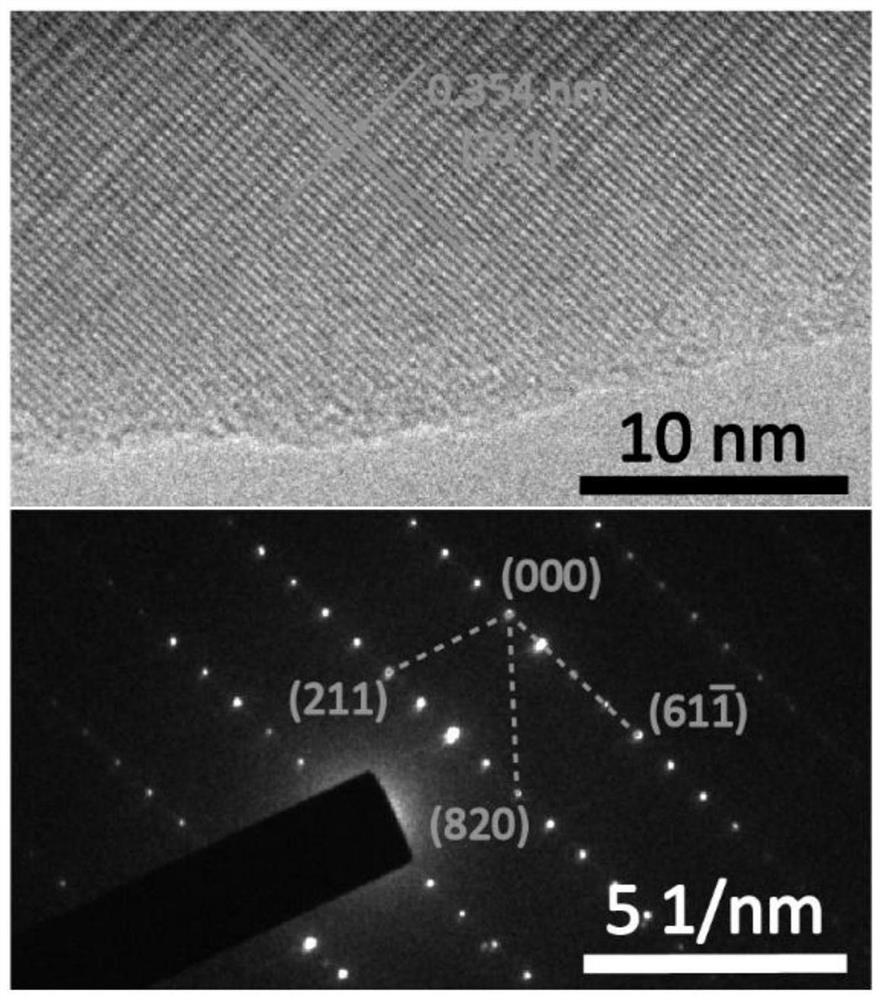
[0064] 2. Material performance characterization and electrochemical performance test: The crystal structure of the material is analyzed by XRD diffractometer (using Rigaku MiniFlex600 diffractometer), and the material can be proved to be tetragonal phase Nb through crystal ...
Embodiment 2
[0067] 1. The difference from Example 1 is: the fuel is glucose, Nb 2 o 5 : WO 3 Molar ratio=7:3, (n niobium+n tungsten):n glucose=1:1.
[0068] 2. Electrochemical properties: Figure 5 For Nb in Example 2 14 W 3 o 44 Electrochemical cycle performance of materials. The prepared electrode material was at 0.2Ag -1 Charge and discharge at current density, the reversible capacity can be maintained at 211.1 mAhg after 200 cycles -1 , The capacity retention rate was 96.0%.
Embodiment 3
[0070] The difference from Example 1 is that the temperature of the self-propagating combustion reaction is 1050° C., the time of the self-propagating combustion reaction is 2 minutes, and the calcination time is 40 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com