Acid-base and photochromic molecular switch and synthesis method thereof
A photochromic and molecular switching technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problems of low yield, expensive raw materials, poor selectivity, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0052] Compound I of the present invention has a molecular structure as follows:
[0053]
[0054] The preparation method and synthetic route of the compound of this example 1 are as follows:
[0055]
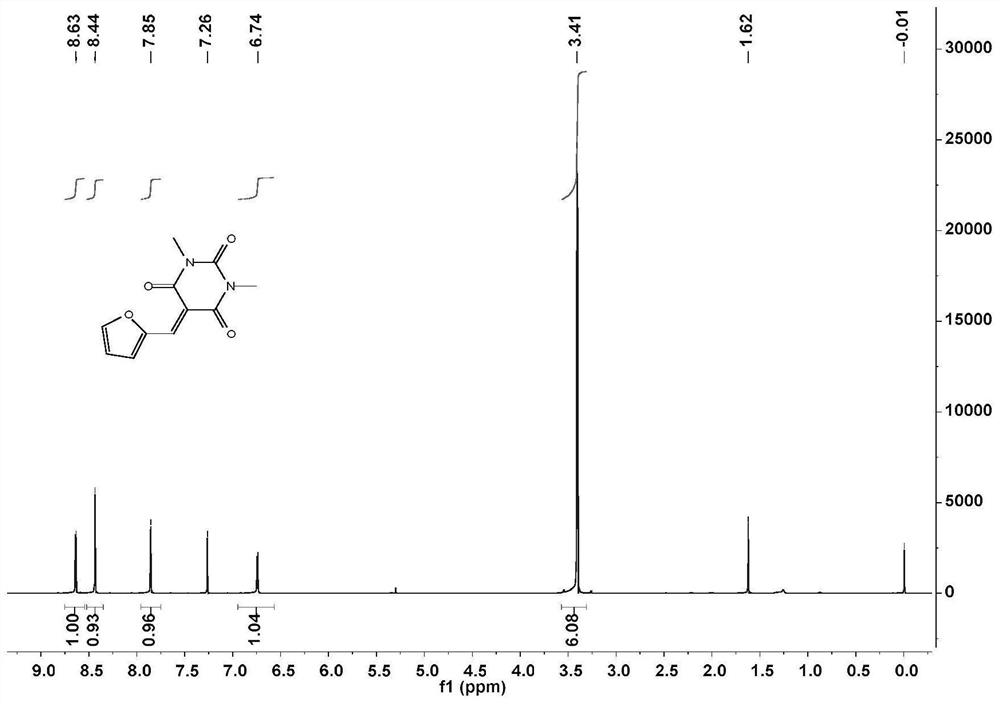
[0056] Add 1,3-dimethylbarbituric acid and furfural to H 2 O middle. The heterogeneous mixture was stirred at room temperature for 2 hours, during which time a yellow precipitate formed. After the reaction (TLC) detection, hexane:ethyl acetate=3:1. The mixture was vacuum filtered to collect the precipitated solid and washed twice with cold water. The collected solid was dissolved in dichloromethane and washed with saturated NaHSO 3 、H 2 O, saturated NaHCO 3 and washed with brine. MgSO for organic layer 4 After drying and filtering, the solvent was removed by a rotary evaporator to obtain a bright yellow powder, whose NMR spectrum was shown in figure 1 .
example 2
[0058] Compound II, its molecular structure is as follows:
[0059]
[0060] The preparation method and synthetic route of the compound of this example 2 are as follows:
[0061]
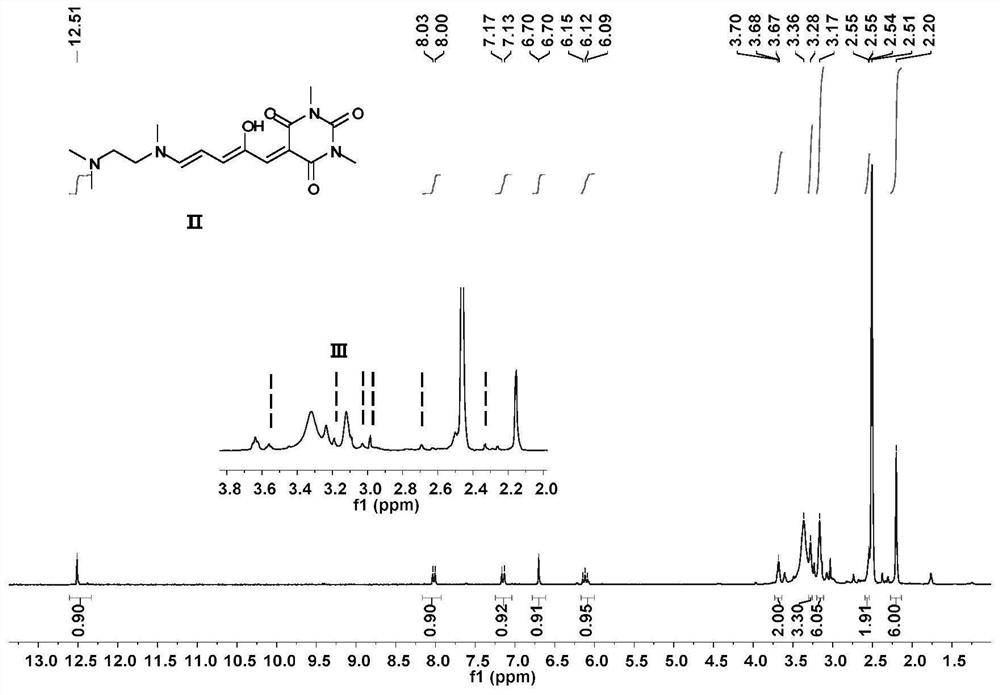
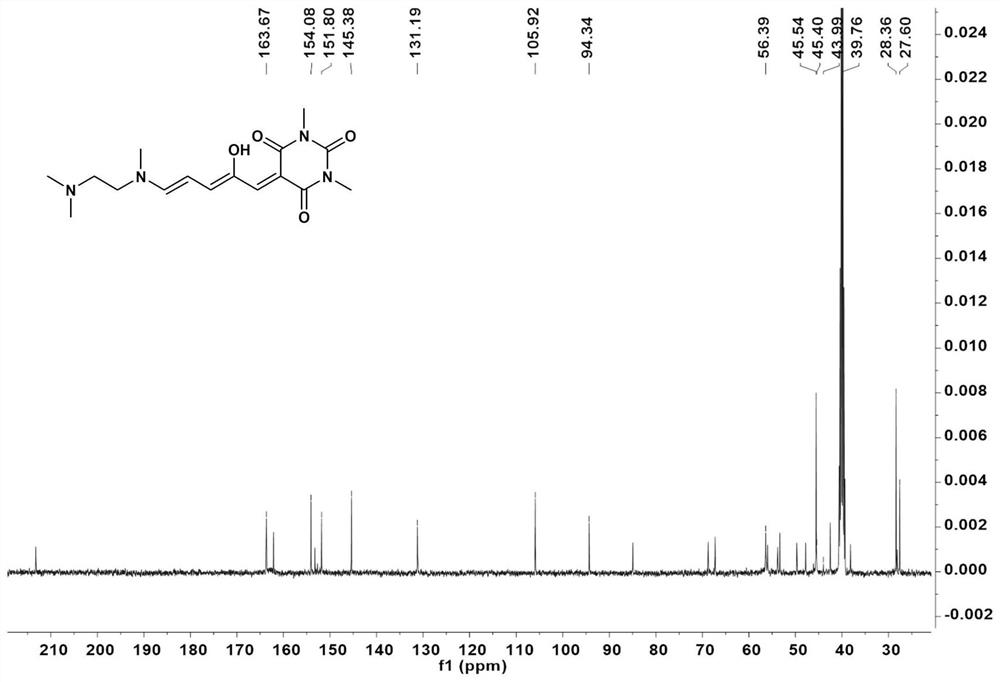
[0062] In the two-necked flask, according to the molar ratio of compound I:N,N,N-trimethylethylenediamine=1:1, add THF sequentially. The mixture was stirred at 23°C for 10 minutes, then cooled at 0°C for 20 minutes. After the reaction was complete, the mixture was filtered to collect solids. The solid was washed with cold ether and dried in vacuo to obtain a green solid whose structural characterization is shown in Figure 2-4 .
example 3
[0064] Compound III, its molecular structure is as follows:
[0065]
[0066] Compound II was recrystallized in acetonitrile at 50°C and left for 2 days to obtain Compound III. For its structural characterization, see Figure 5-7 , whose IR see Figure 13 , whose XRD see Figure 14 .
[0067]
[0068] The crystal data obtained after recrystallization of compound II are shown in Table 1-2 below.
[0069] Table 1 Crystal parameters
[0070]
[0071]
[0072] Table 2 Bond length between atoms
[0073]
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com