Method for loading drug on non-denatured human H ferritin
A ferritin, non-denaturing technology, applied in the field of ferritin-encapsulated drugs, can solve the problems of difficult industrial transformation, unstable ferritin-drug complex, long incubation time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
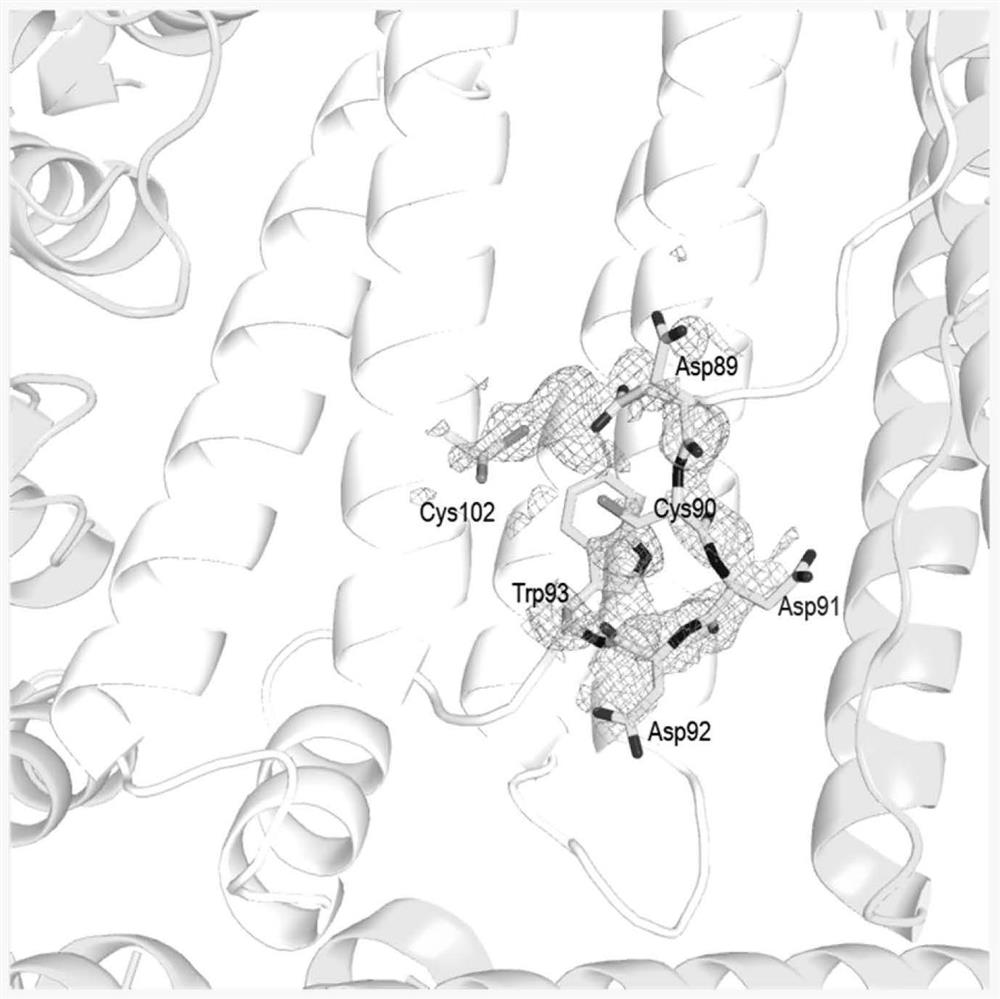
[0062] Example 1 Structural Analysis of the Small Molecule Drug Doxorubicin (Dox) Channel on the HFn Protein Shell
[0063] In this embodiment, the following method steps are used to analyze the structure of the channel of the small molecule drug doxorubicin (Dox) on the HFn protein shell:
[0064] Step (1) Construction of HFn expression plasmid: After the DNA sequence of HFn (shown in SEQ ID No: 2, its amino acid sequence is shown in SEQ ID No: 3) through the whole gene synthesis (Generay, Shanghai), use NdeI and It was digested with BamHI restriction endonuclease and cloned into the Escherichia coli (E.coli) expression vector pET22b (+) plasmid (Novagen) with NdeI and BamHI restriction enzyme cutting sites, and the sequence was confirmed to be correct by DNA sequencing.
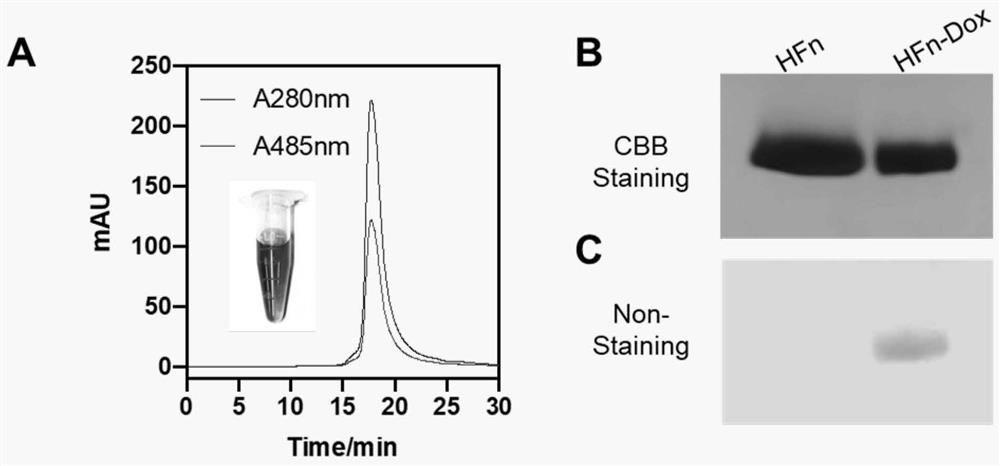
[0065] Step (2) expression and purification of HFn: the above-mentioned obtained plasmid is transferred into E.coli BL21 (TransGen) expression strain, and the Escherichia coli after transformation grows ove...
Embodiment 2
[0069] Example 2 Mutation verification of Dox drug loading channel on the surface of HFn protein
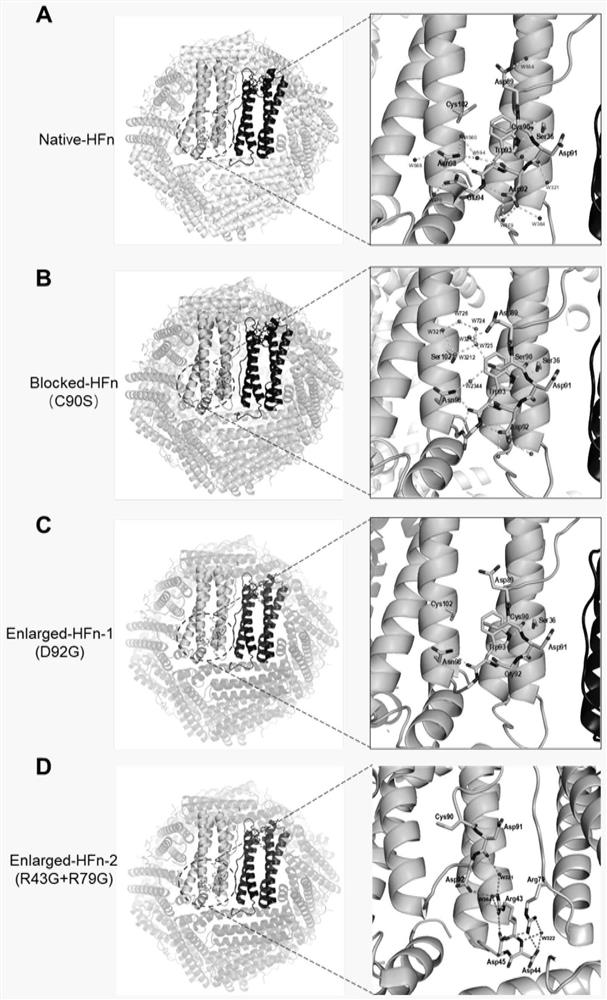
[0070]In order to verify the existence of Dox drug loading channel on the surface of HFn protein, we synthesized a series of mutants of HFn. Including the HFn C90S / C102S / C130S (SEQ ID No: 4) mutant demonstrating the existence of the channel, and Enlarged-HFn-1 (D92G) (SEQ ID No: 5), Enlarged-HFn-2 (R43G+R79G ) (SEQ ID No: 6) and Block-HFn(C90S) (SEQ ID No: 7) with closed channel. On the HFn protein shell, the residues in the 89-91 part can shift sideways, and Cys90 and Asp91 have almost no interaction with the surrounding residues or water molecules, and the flexibility is particularly high. The region between C90S and C102S of the C90S / C102S / C130S mutant forms a hydrogen bond network formed by adjacent residues and bound water molecules. This hydrogen bond network relatively fixes the conformation of residues 89-91, making 89 The -91 residue is very stable and inhibits the lat...
Embodiment 3
[0074] Example 3 Promotes the loading of small molecule drugs by controlling the temperature
[0075] Based on the analysis results of the ferritin structure, we speculate that the heating method can promote the further opening of the ferritin drug channel, which is more conducive to drug loading.
[0076] method:
[0077] 1. Temperature gradient experiment
[0078] 15% glycerol, 2 mg WT-HFn, 0.75 mg doxorubicin Dox, and 50 mM Trisbuffer were added to 1 mL reaction system to make up to 1 mL, pH 8.0. Incubate for 4 hours at 4°C, 25°C, 37°C, 42°C, 50°C, 60°C, 65°C, and 72°C, respectively. Centrifuge at 12000rpm for 10min at 4°C, take the supernatant, and send it to the desalting column Hiprep TM 26 / 10 Desalting treatment removed free doxorubicin, and obtained HFn-Dox ferritin doxorubicin samples. The concentrations of HFn and Dox in the HFn-Dox samples were determined to calculate the Dox loading and HFn protein yield.
[0079] 2. Time Gradient Experiment
[0080] 15% gly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com