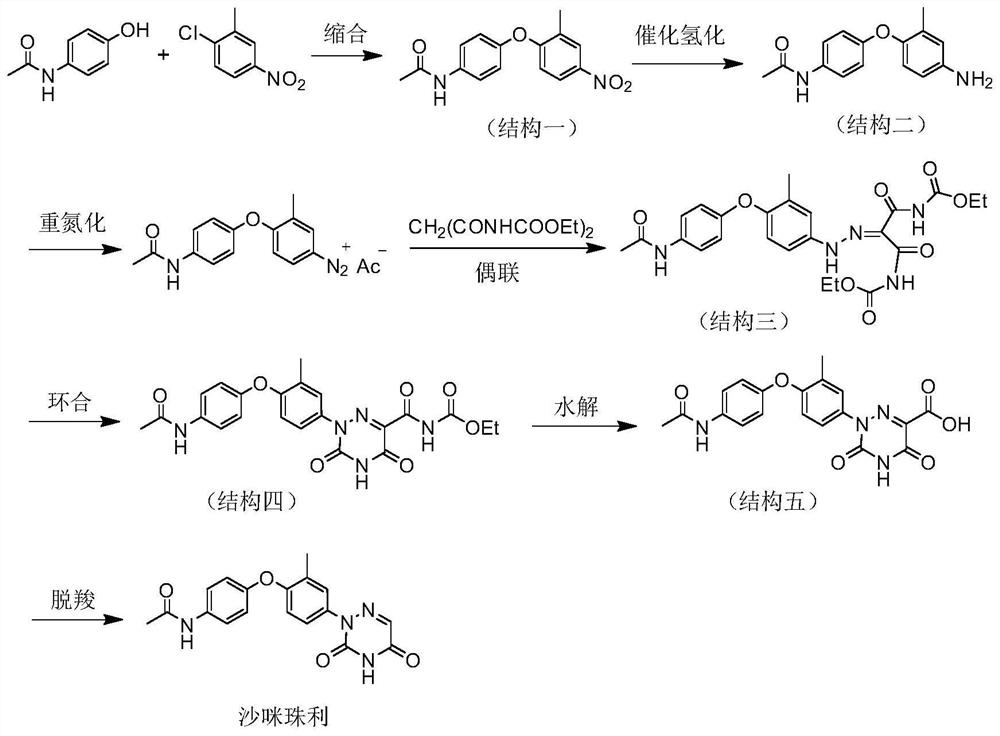
Preparation method of Ethanamizuril bulk drug
A technology of samizuril and raw materials, which is applied in the field of preparation of samizuril raw materials, can solve the problems of samizuril with many impurities, unfavorable samizuril application, and difficult purification, and achieve mild hydrolysis conditions, The effect of good industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
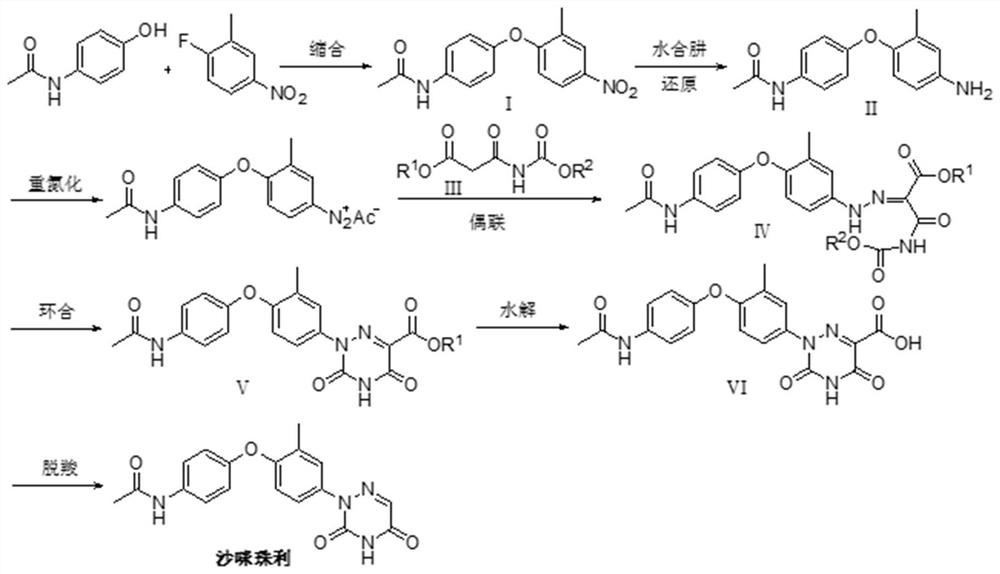
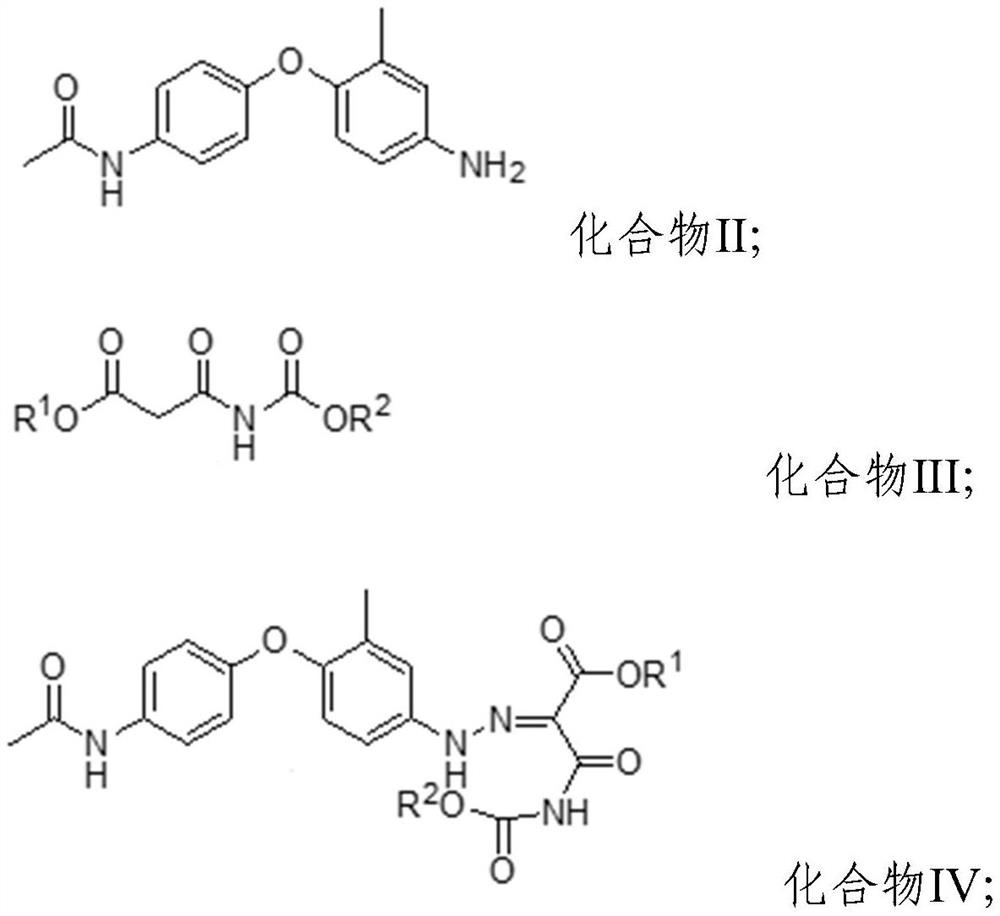
[0040] The present embodiment provides a kind of preparation method of samizuril crude drug, and its synthetic route is as follows figure 2 As shown, it specifically includes the following steps:
[0041] Synthesis of compound I:
[0042] At room temperature, in a 500mL round bottom flask, add solvent DMF (250mL), potassium carbonate (64g), paracetamol (61g, 0.40mol), after stirring for one hour, add 2-fluoro-5-nitrotoluene (62g, 0.36mol), heated to 140°C in an oil bath, stirred and reacted for 5h, removed the oil bath, stirred to cool down, added water (500mL), a large amount of light yellow solid precipitated, stirred for ten minutes, and suction filtered to obtain a solid product, water After washing and drying at 80°C, compound I was obtained, 103 g of light yellow powder, with a yield of 99.5%.
[0043] Recrystallized to obtain colorless needle crystals, melting point: 164.2-164.6°C. 1 H NMR (400MHz, DMSO-d 6 )δ10.06(s,1H),8.20(d,J=2.8Hz,1H),8.02(dd,J=9.1,2.9Hz,1H),7...
Embodiment 2
[0059] The present embodiment provides a kind of preparation method of samizuril crude drug, specifically comprises the following steps:
[0060] Synthesis of compound I:
[0061] At room temperature, in a 500mL round bottom flask, add the solvent DMAC (250mL), sodium carbonate (49g), paracetamol (59g, 0.39mol), after stirring for one hour, add 2-fluoro-5-nitrotoluene (62g, 0.36mol), heated to 140°C in an oil bath, stirred and reacted for 5h, removed the oil bath, stirred to cool down, added water (500mL), a large amount of light yellow solid precipitated, stirred for ten minutes, and suction filtered to obtain a solid product, water After washing and drying at 80°C, compound I was obtained, 102 g of light yellow powder, with a yield of 99.0%.
[0062] Synthesis of compound II:
[0063] In the 1000mL round bottom flask, add anhydrous methanol (500mL), ferric chloride (0.5g), gac (5g), sodium hydroxide (0.5g), compound I (100g, 0.35mol), in the oil bath, heat to Reflux, unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com