Preparation method and screening method of salmonella PagC protein monoclonal antibody
A technology of monoclonal antibodies and screening methods, applied in the direction of antibacterial immunoglobulin, immunoglobulin, chemical instruments and methods, etc., can solve serious cross-reaction of Enterobacteriaceae bacteria, insufficient sensitivity of serum samples, and complex antigenic epitopes And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
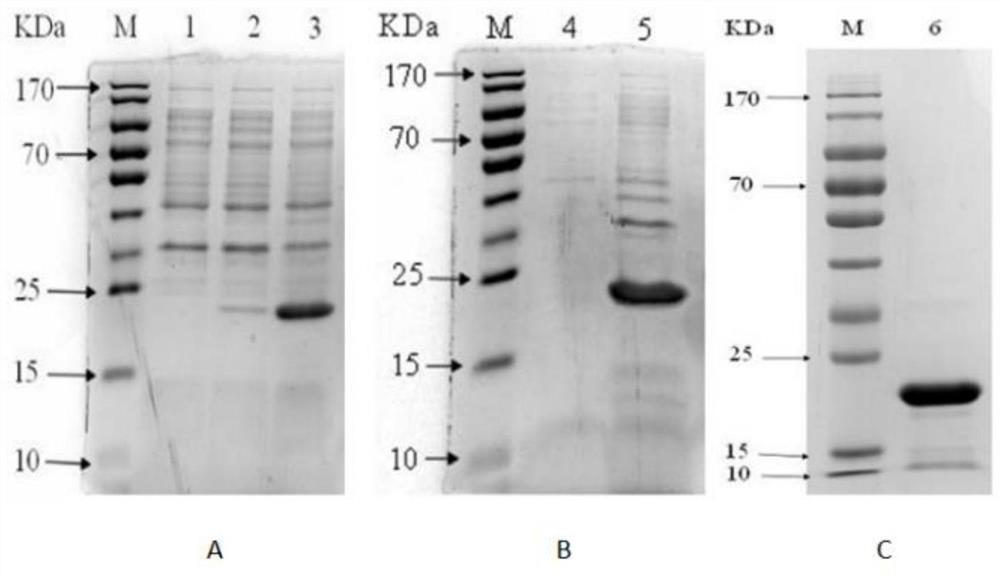
[0035] see Figure 1-2 , the present invention provides a technical solution: preparation of antigen,
[0036] 1.1 Expression, purification and renaturation of PagC protein
[0037] The Salmonella PagC recombinant expression strain pET-28a-PagC was cultured to the logarithmic phase with LB (adding kanamycin with a final concentration of 50 ng / mL); Lactoside, Isopropylβ-D-Thiogalactoside) inducer, 37 ℃ for 3 hours of shaking culture; the induced cells were washed three times with sterilized PBS, and finally resuspended in PBS, then ultrasonically disrupted the cells (power 200W, work 5s interval 8s); 1 hour later, centrifuge at 4°C for 30 minutes, take the supernatant and precipitate for SDS-PAGE, determine the expression form of the protein, and then perform a large amount of expression, and purify according to the purification process of the inclusion body protein. It is confirmed that the purified protein needs to be further refolded. This time, the gradient dialysis method ...
Embodiment 2
[0043] see Figure 3-5 , the present invention provides a technical solution: the preparation of PagC protein monoclonal antibody,
[0044] 2.1 Animal immunity
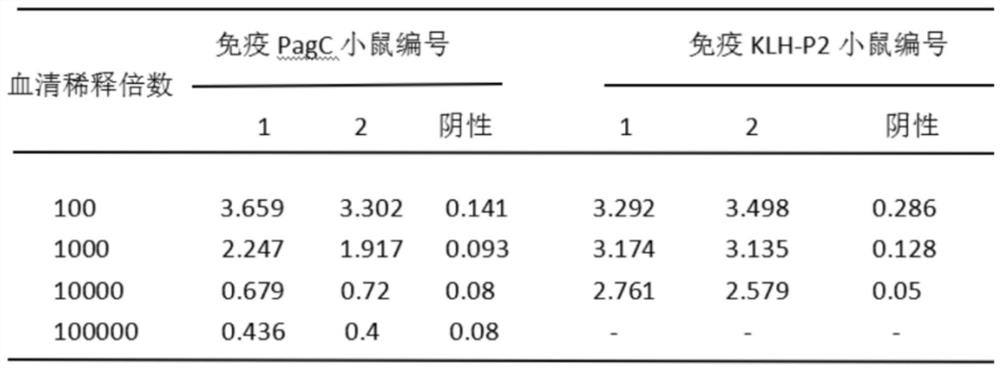
[0045] Select SPF grade female Balb / c mice aged 6-8 weeks, and inject 50ug / 0.3mL subcutaneously on the back after emulsification with purified protein PagC for initial vaccination; 21 days later, inject 50ug / 0.5mL subcutaneously and intraperitoneally after antigen emulsification / mouse; 10 days after the second immunization, blood was collected to separate the serum, and the mouse titer was determined by the established indirect ELISA, and the mouse with the highest titer and greater than 104 was selected for intraperitoneal injection of 100ug / 0.5mL / antigen; 3 days later, splenocytes were collected for fusion . KLH-P2 immunized mice, the immunization dose was 100ug / 0.5mL / mouse, other methods refer to the above immunization method.
[0046] After the second immunization, the mouse titer reached 104, and splenocytes...
Embodiment 3
[0058] see Figure 6-7 , the present invention provides a technical solution: screening of specific monoclonal antibodies,
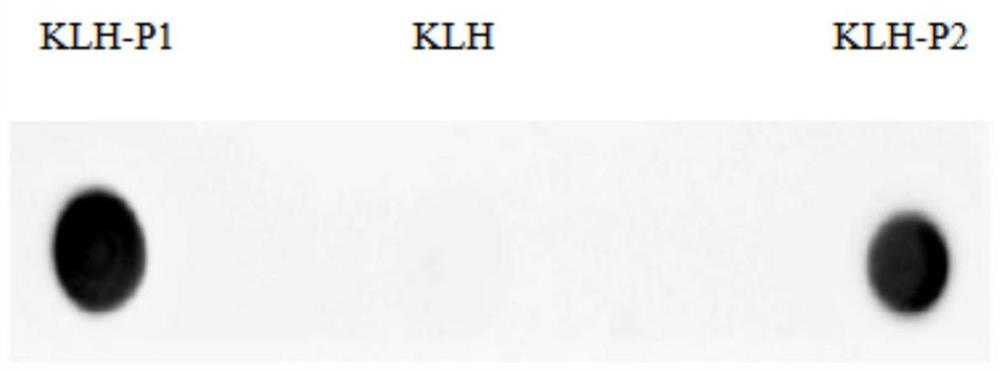
[0059] 3.1 Preliminary identification of B cell epitopes of Salmonella PagC protein monoclonal antibody by Dot-Blot
[0060] Add 3ug PagC, KLH-P1, KLH-P2, and KLH dropwise on the NC membrane, overnight at 4°C; add 5% skimmed milk to block at room temperature for 2h; wash with PBST 3 times, 5min / time; add positive hybridoma cell supernatant, Incubate at room temperature for 1 hour; wash with PBST 3 times, 5 minutes each time; add goat anti-mouse HRP enzyme-labeled secondary antibody diluted 1:5000, incubate at room temperature for 1 hour; wash with PBST 3 times, 5 minutes each time; develop color with DAB in the dark for 5-10 minutes, Rinse the NC membrane in distilled water to stop the color development, and take it out to dry.
[0061] The results showed that the J cell strain had an obvious reaction with KLH-P1, but had no reaction with KLH and KLH-P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com