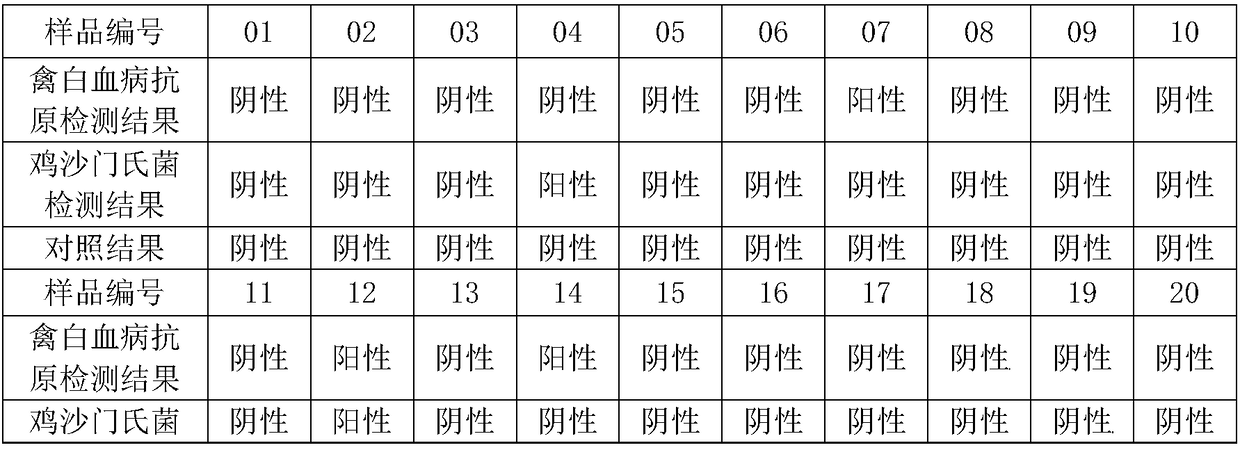
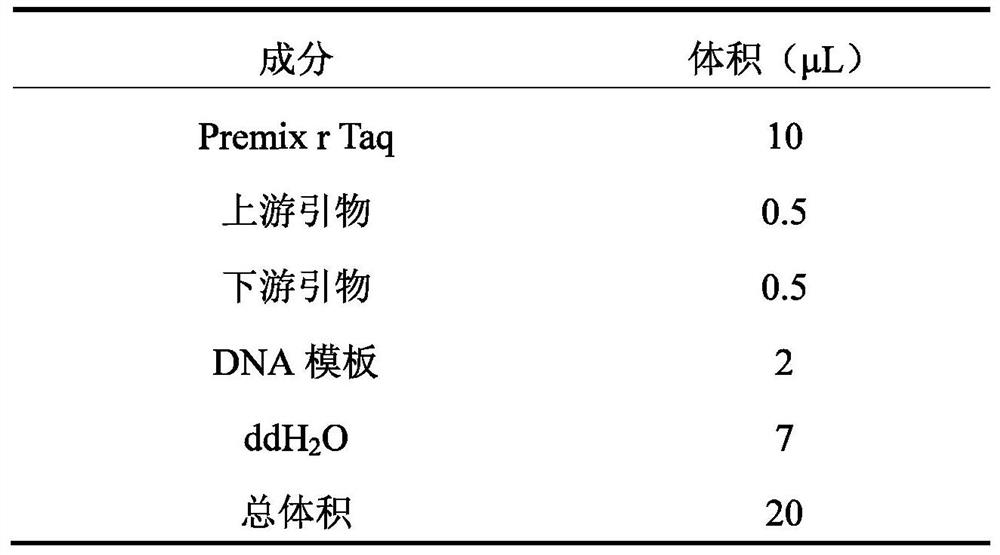
Patents
Literature
31 results about "Salmonella antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
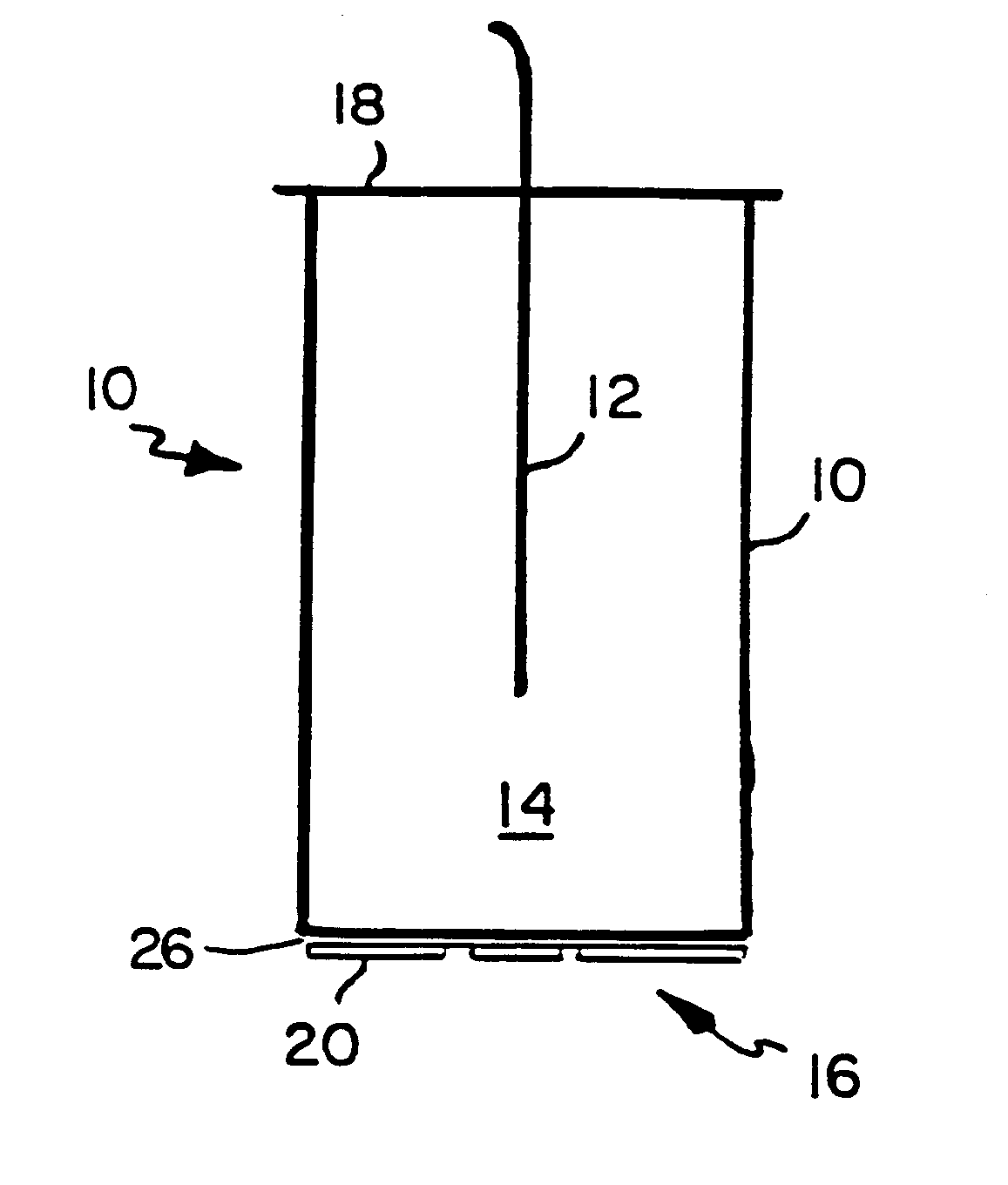
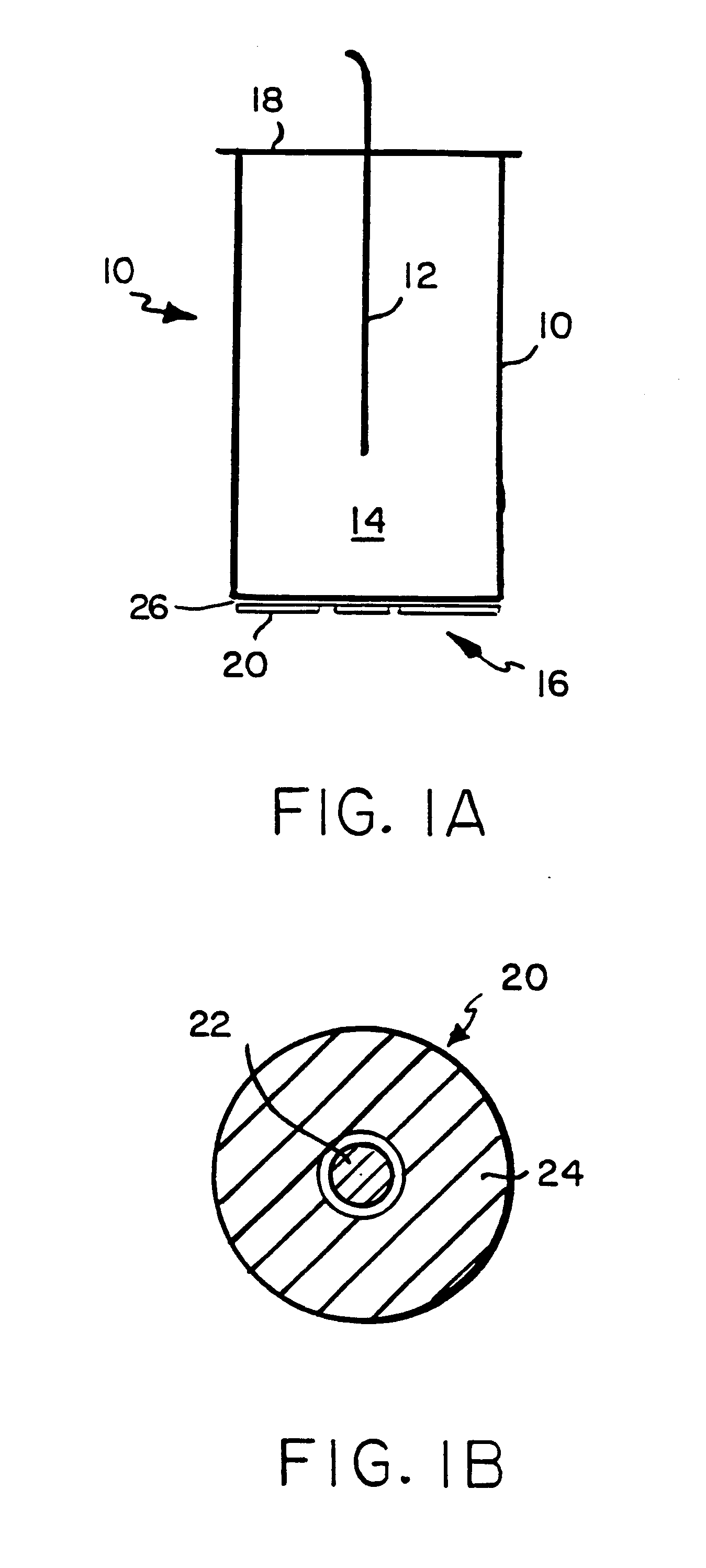
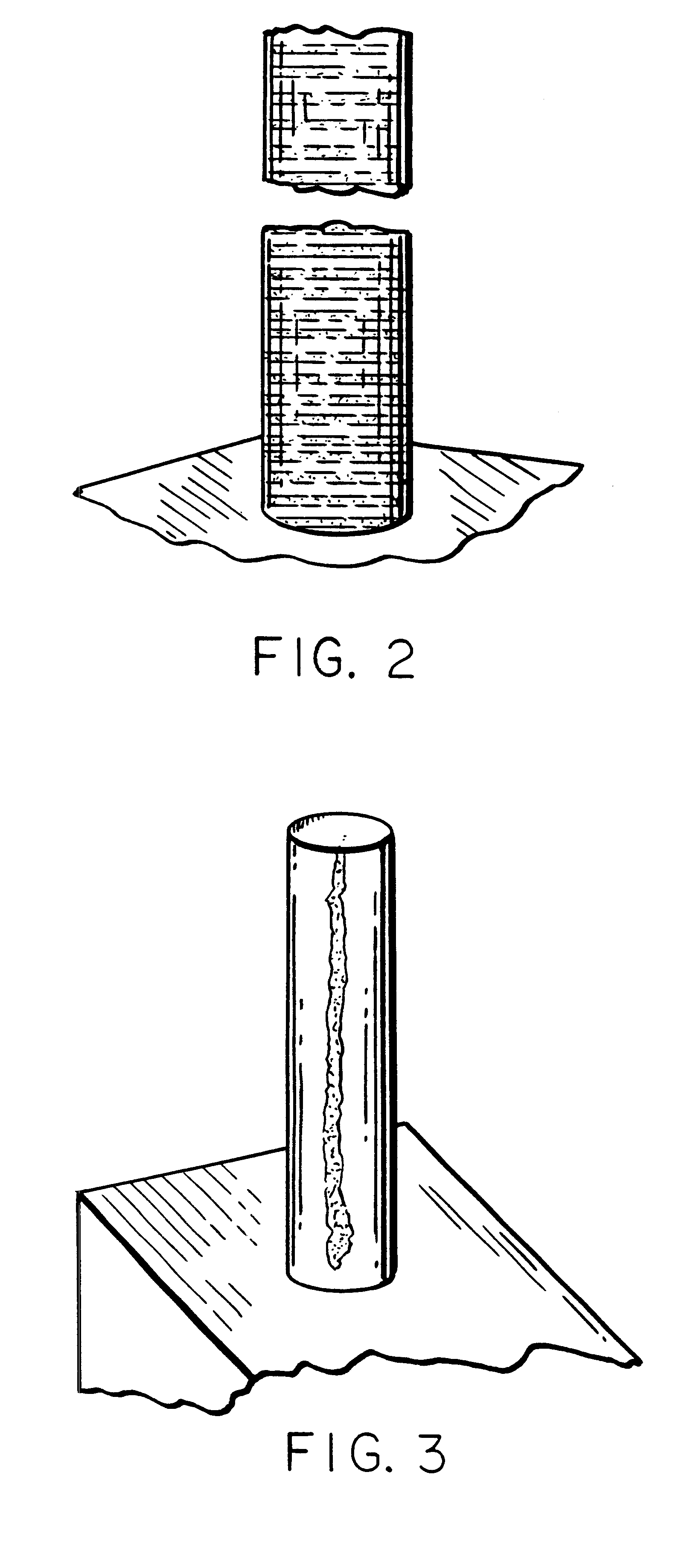
Acoustic standing-wave enhancement of a fiber-optic salmonella biosensor
InactiveUS6391653B1Bioreactor/fermenter combinationsBiological substance pretreatmentsMicrosphereTest chamber
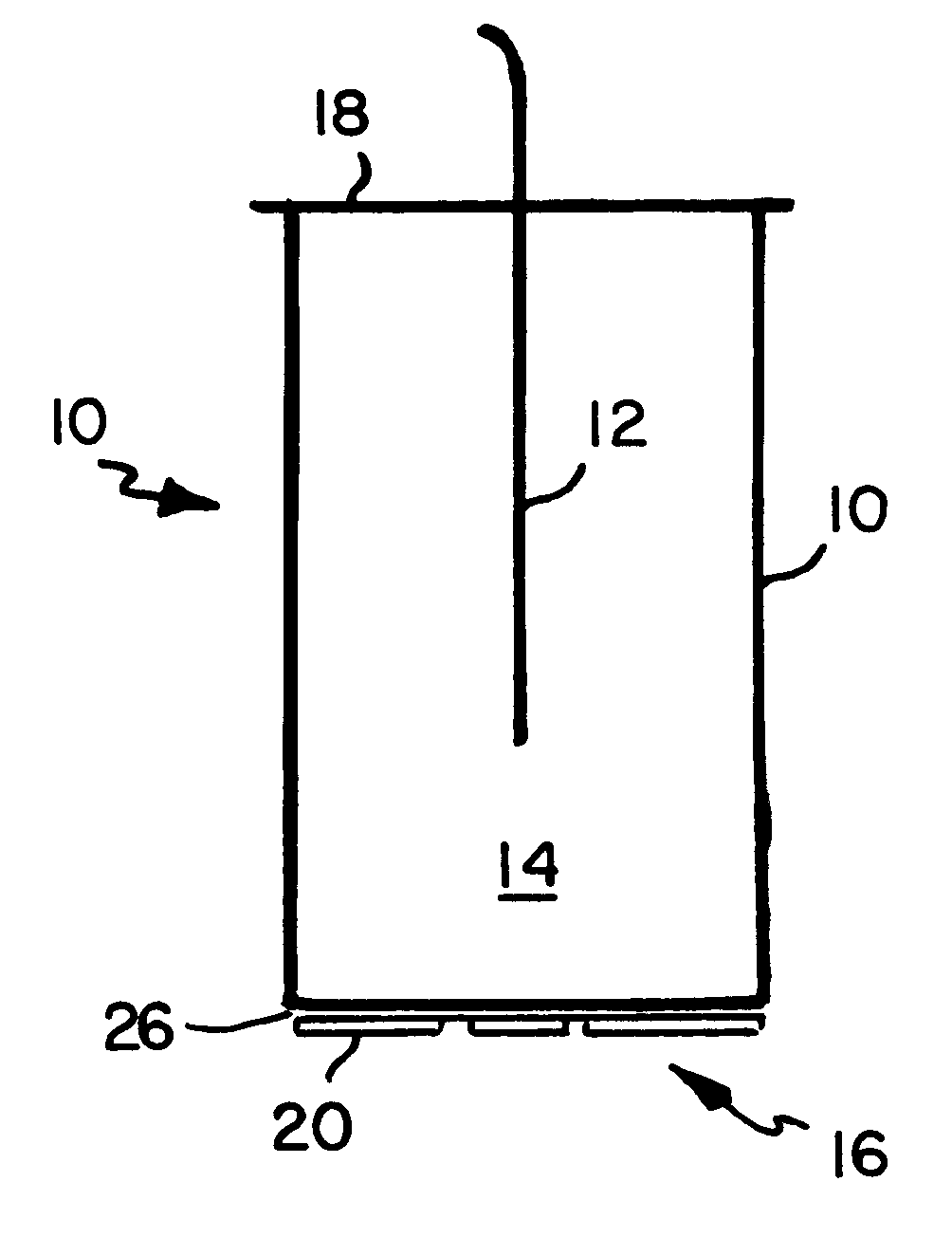
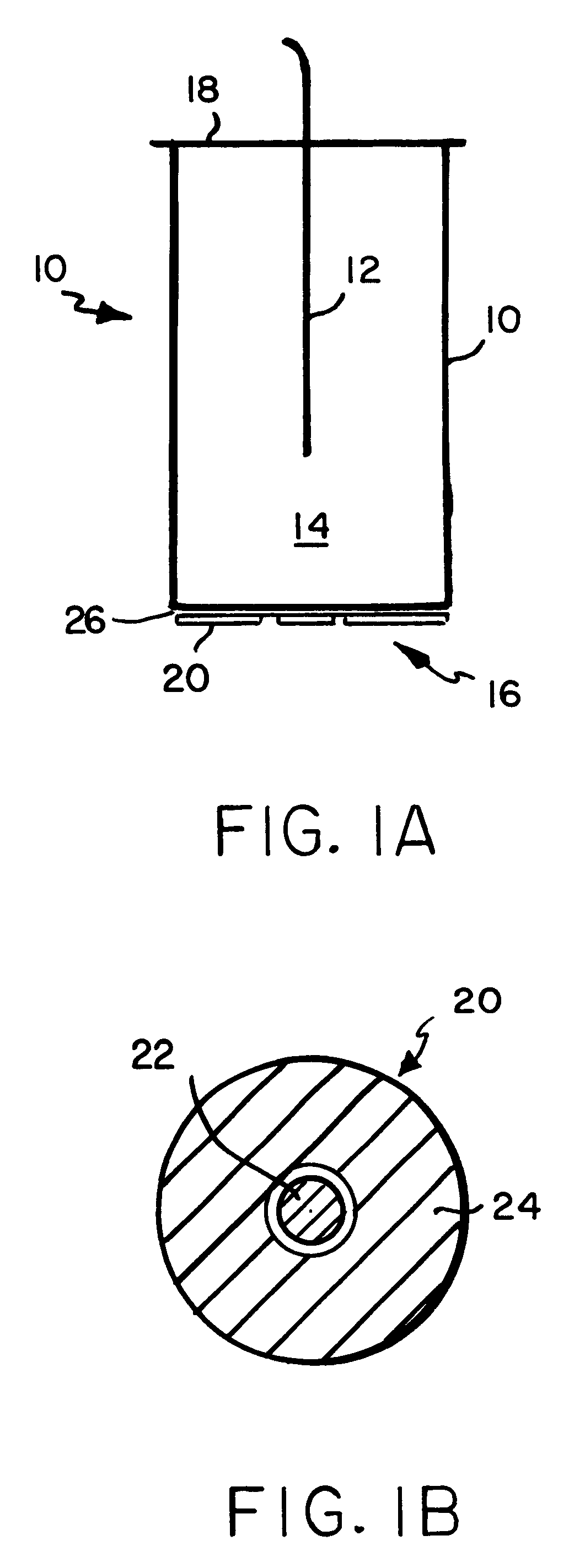
A fluorescent fiber-optic biosensor system using ultrasonic concentration of particles and cells for the detection of Salmonella typhimurium. A biosensor test chamber serves as an ultrasonic standing-wave cell that allows microspheres or cells to be concentrated in parallel layers or in a column along the axis of the cell. A fiber probe along the axis delivers laser excitation to fluorescent-labeled antibodies of Salmonella and collects the fluorescent signal. The Salmonella-antibody complexes are moved acoustically to the axis of the cell, increasing the fluorescent signal. Alternatively, the Salmonella-labelled antibody complexes attach to unlabeled antibodies that have been immobilized on the surface of polystyrene microspheres. This entire structure can be manipulated acoustically and the increase in the fluorescent signal, which can be an order of magnitude, indicates the presence of Salmonella.
Owner:BOARD OF GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND & PROVIDENCE PLANTATIONS
Method for sensitively, simply and conveniently detecting bacteria
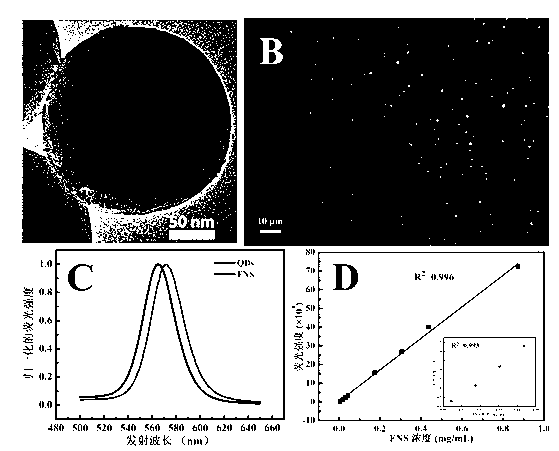
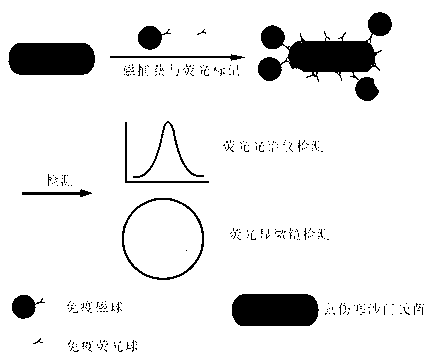
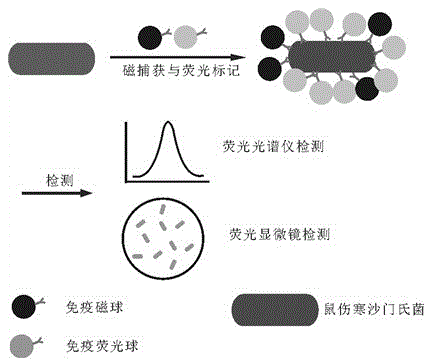
InactiveCN102841198ASimple stepsEasy to detectFluorescence/phosphorescenceSalmonella kielFluorescence microscope
The invention relates to a method for detecting bacteria. The method comprises the steps as follows: coupling magnetic nanospheres and fluorescent nanospheres with mouse typhus salmonella antibodies so as to obtain mouse typhus salmonella-targeted immunologic magnetic spheres and immunologic fluorescent spheres, and adding the mouse typhus salmonella-targeted immunologic magnetic spheres and immunologic fluorescent spheres into a detection system so as to realize magnetic capture and fluorescent labeling to the mouse typhus salmonella simultaneously. About 10 CFU / mL of the mouse typhus salmonella can be detected through observation of a fluorescent microscope; and a good quantitative relation is obtained through detection of a fluorescence spectrophotometer, the linear range is 105-107 CFU / mL, and R2 is equal to 0.9994. The whole detection process is very simple, high in sensitivity and strong in specificity, and can be finished detection within 1.5 h.
Owner:WUHAN UNIV
Acoustic standing-wave enhancement of a fiber-optic Salmonella biosensor
A fluorescent fiber-optic biosensor system using ultrasonic concentration of particles and cells for the detection of Salmonella typhimurium. A biosensor test chamber serves as an ultrasonic standing-wave cell that allows microspheres or cells to be concentrated in parallel layers or in a column along the axis of the cell. A fiber probe along the axis delivers laser excitation to fluorescent-labeled antibodies of Salmonella and collects the fluorescent signal. The Salmonella-antibody complexes are moved acoustically to the axis of the cell, increasing the fluorescent signal. Alternatively, the Salmonella-labelled antibody complexes attach to unlabeled antibodies that have been immobilized on the surface of polystyrene microspheres. This entire structure can be manipulated acoustically and the increase in the fluorescent signal, which can be an order of magnitude, indicates the presence of Salmonella.
Owner:BOARD OF GOVERNORS OF HIGHER EDUCATION STATE OF RHODE ISLAND & PROVIDENCE PLANTATIONS THE
ELISA kit for detecting Salmonella pullorum antibody
ActiveCN103995126AReduce stressImprove featuresBiological material analysisBiological testingAntigenElisa kit
An ELISA kit for detecting a Salmonella pullorum antibody is established by screening Salmonella pullorum dominant antigen GroEL through an immunoprecipitation technology, expressing GroEL recombinant protein through utilizing a prokaryotic expression vector, and utilizing an antigen protein. The kit can reduce the response of chicken in the detection process of the Salmonella pullorum antibody, and can improve the detection specificity and the sensitivity.
Owner:WENS FOOD GRP CO LTD
ELISA kit for detecting salmonella antibody
InactiveCN106290918AImprove featuresGood repeatabilityDisease diagnosisBiological testingElisa kitGenus Orthobunyavirus
The invention discloses an ELISA kit for detecting a salmonella antibody. The kit comprises a solid-phase carrier coated with recombinant protein PagC, an enzyme-labeled antibody, salmonella negative serum and positive serum. The ELISA kit and method for detecting the salmonella antibody can be widely applied to salmonella, and application range is wide. The kit has high specificity and repeatability, the influence of temperature on reaction plates is small, and stability is high. Compared with an ELISA method with polysaccharide antigen as detection antigen, the method has the advantages that the occurrence rate of false positivity can be reduced to the maximum, and interference of other bacterial antigens in enterobacteriaceae on detection is avoided. Compared with a slide agglutination antigen detection method mostly adopted clinically, the method has the advantages that sensitivity and accuracy are higher, and detection throughput is increased greatly.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Kit used for detecting swine salmonella antibody
The invention provides a kit used for detecting swine salmonella antibody. The kit comprises antigen protein; the antigen protein is fljB protein, and the amino acid sequence of the antigen protein isrepresented by SEQ ID NO.3. The fljB protein is excellent in antigenicity, and high in specificity, purity, and yield; in the kit, the antigen protein coating concentration is 20<mu>g / ml, and negative and positive serum dilution ratio is 1:200. The kit can be used for swine salmonella fljB antibody rapid detection of clinical samples, is capable of solving a problem in the prior art that there isno single antigen salmonella infection diagnosis method, reducing failure detection rate, and increasing precision. A sensitive detection method is provided for swine salmonella epidemiological investigation in China, and the kit can be used for early diagnosis of swine salmonella infection.
Owner:CHINA AGRI UNIV
Test paper for salmonella and preparation method of test paper
InactiveCN106018799APracticalStrong specificityBiological material analysisNitrocelluloseEngineering
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Non-diagnostic purpose method for quantitatively detecting Salmonella pullorum and Salmonella gallinarum
InactiveCN108362872ARealize quantitative detectionThe detection method is simpleMaterial analysisSalmonella GallinarumMicrosphere
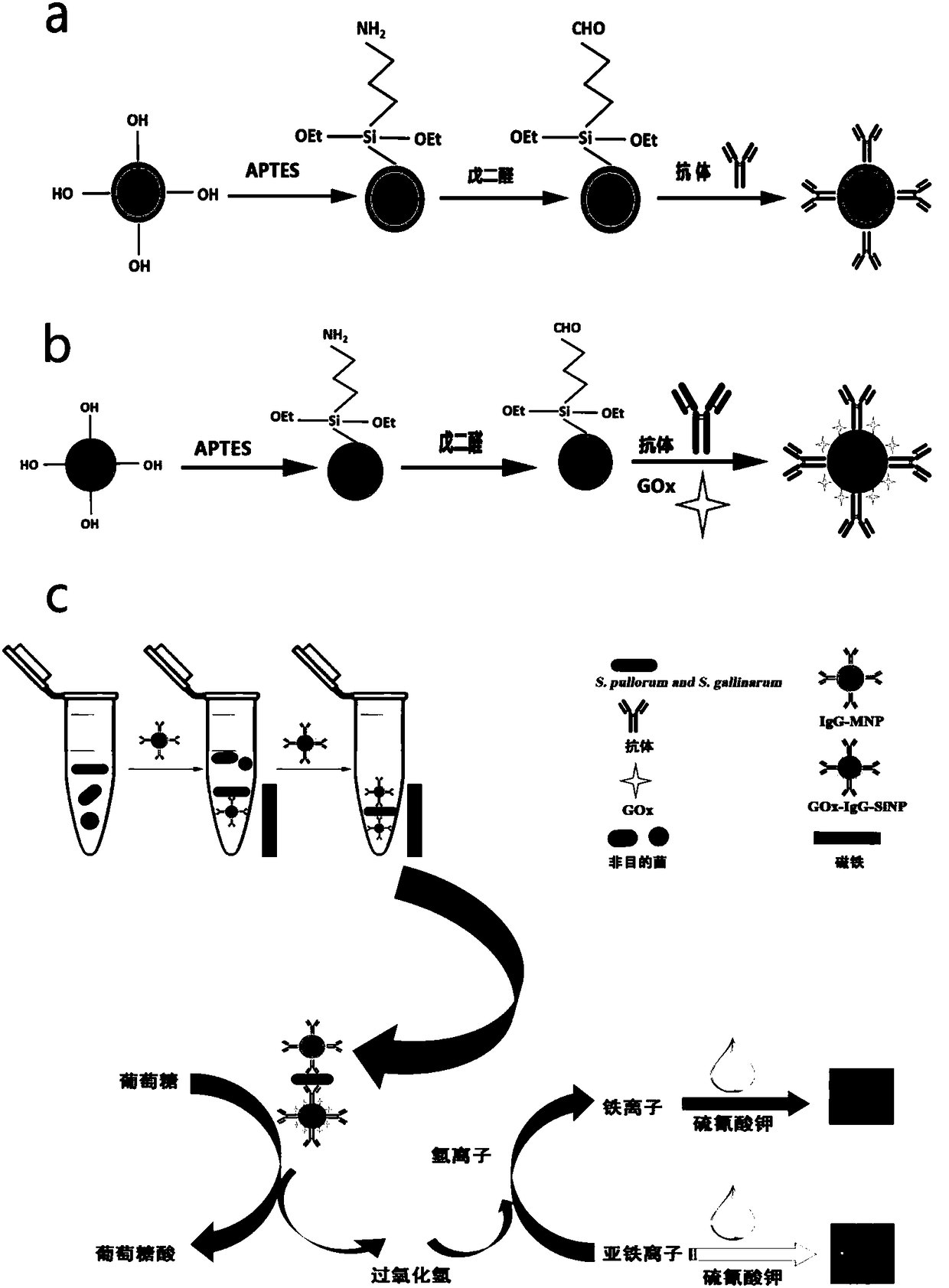
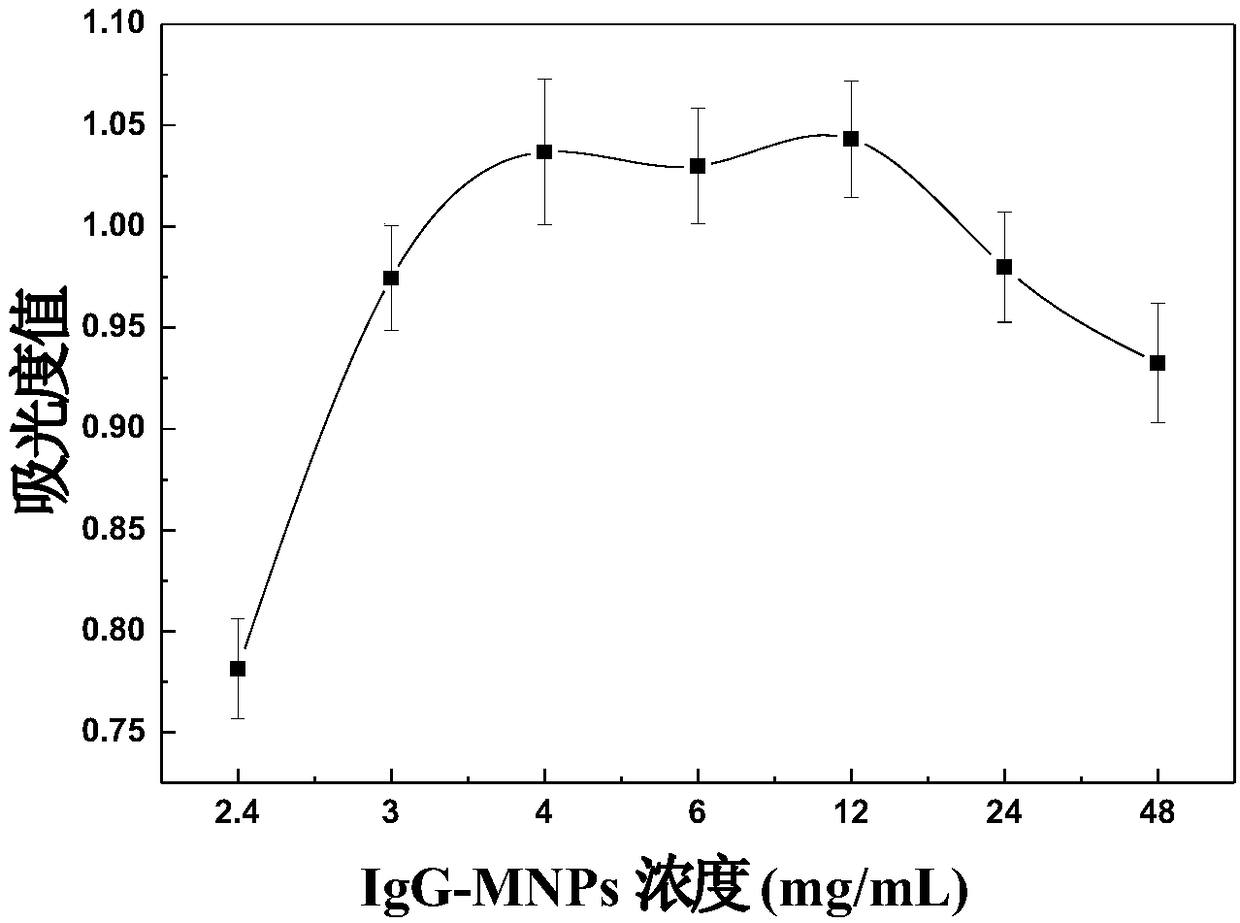
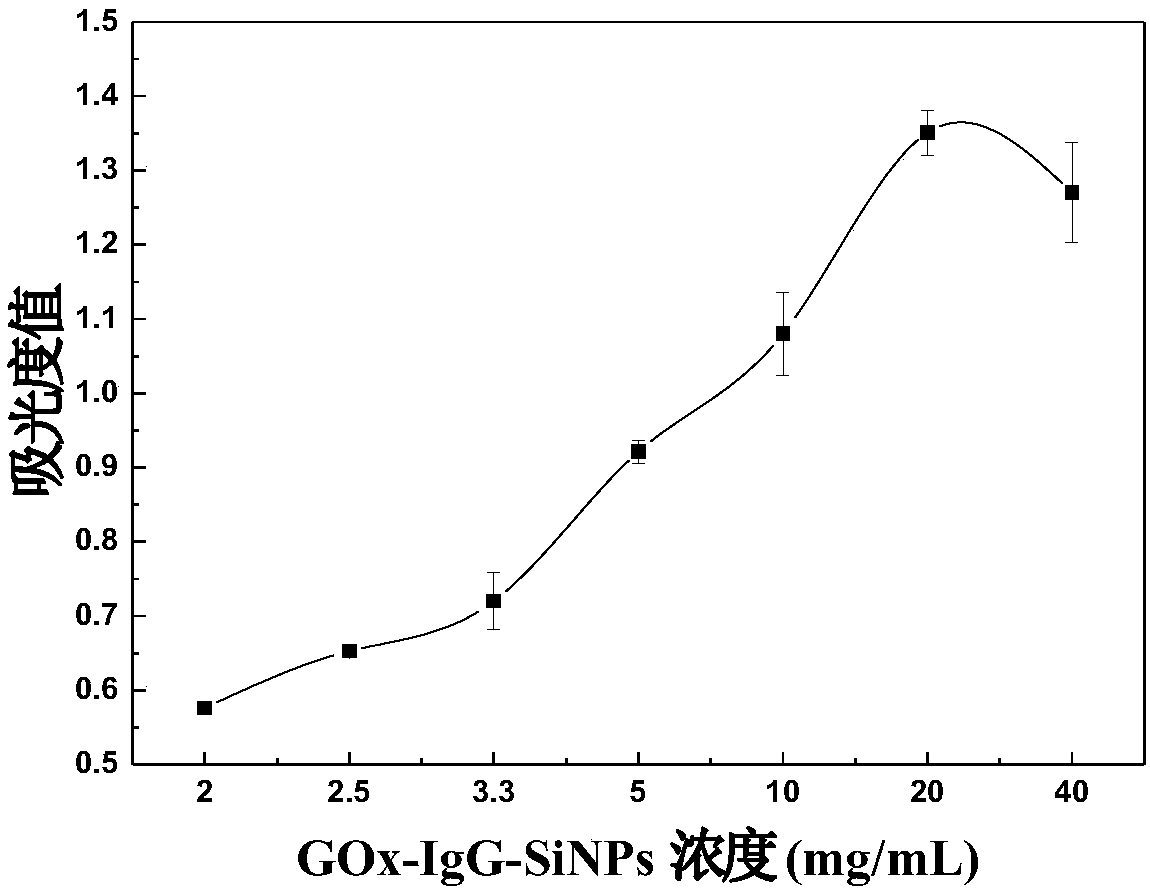
The invention discloses a non-diagnostic purpose method for rapidly detecting Salmonella pullorum and Salmonella gallinarum. The detection method comprises the following steps: preparing Salmonella pullorum and Salmonella gallinarum antibody labeled coupled magnetic nano-particles (IgG-MNPs) and antibody and enzyme double labeled silica nano-particles (GOx-IgG-SiNPs), forming an immune compound IgG-MNPs / S having a sandwich structure, S. Pullorum and S. Gallinarum GOx-IgG-SiNPs, when the target bacteria to be detected exist, performing an enzymatically catalyzed reaction, allowing the obtainedreaction product and a color developer to form a complex product, and detecting the absorbance value of the obtained system to obtain the concentrations of the Salmonella pullorum and Salmonella gallinarum in order to realize the quantitative detection of the Salmonella pullorum and Salmonella gallinarum. A cascade reaction triggered by glucose oxidase (GOx) is combined with an Fe<3+>-SCN<-> colordevelopment system to realize the quantitative detection of Salmonella pullorum and Salmonella typhimurium. The detection method has the advantages of convenience, fastness, easiness in operation, good sensitivity, good specificity and good stability.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
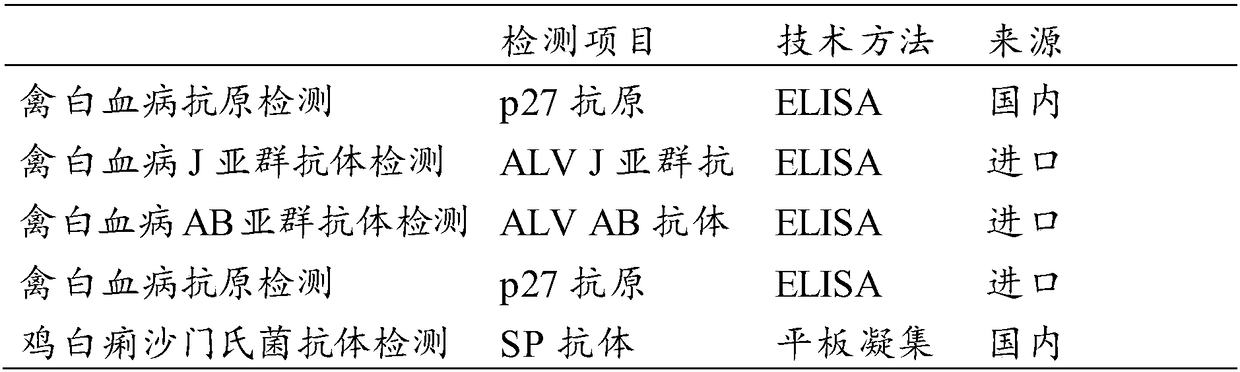
Kit for simultaneously detecting avian leukosis virus antibody and salmonella pullorum antibody
The invention relates to the technical field of biology and in particular relates to a kit for simultaneously detecting an avian leukosis virus antibody and a salmonella pullorum antibody. An antigencombination of the kit comprises p27 protein, gp85 protein and GroEL-delta8-1 protein. Avian leukosis virus capsid protein p27, prokaryotic expression protein of envelope protein gp85, and prokaryoticexpression protein of truncated GroEL-delta8-1 of a salmonella pullorum dominant antigen are used as a coating antigen to develop an ELISA (Enzyme-linked Immunosorbent Assay) kit capable of simultaneously detecting the avian leukosis virus antibody and the salmonella pullorum antibody. Compared with a current common kit for independently detecting avian leukosis or pullorum disease, the kit provided by the invention has the effect of simultaneously detecting the avian leukosis or the pullorum disease, and the detection and purification work of the avian leukosis and the pullorum disease can be extremely alleviated.
Owner:CHINA AGRI UNIV
Kit for simultaneously detecting avian leukosis virus antibody and salmonella pullorum antibody
ActiveCN108709995AWork lessEase the work of decontaminationMaterial analysisLeucosisAvian leukosis viruses
The invention relates to the technical field of animal epidemic disease detection and in particular relates to a kit for simultaneously detecting an avian leukosis virus antibody and a salmonella pullorum antibody. The kit comprises: P27 protein, GroEL-delta8-1 protein, an enzyme labeled second antibody, BSA (Bovine Serum Albumin), coating liquid, confining liquid, a color developing solution anda stopping solution. The invention develops an indirect ELISA (Enzyme Linked Immunosorbent Assay) kit capable of simultaneously detecting the avian leukosis virus antibody and the salmonella pullorumantibody by taking prokaryotic expression protein of a conserved region P27 of an avian leukosis virus and prokaryotic expression protein of truncated protamine GroEL-delta8-1 of a dominant antigen GroEL of salmonella pullorum as coating antigens; the kit provided by the invention can be used for simultaneously detecting avian leucosis and pullorosis; chickens which are detected to be positive areeliminated so that the aim of simultaneously purifying is realized and the clinical detection and purification work is extremely alleviated.
Owner:CHINA AGRI UNIV
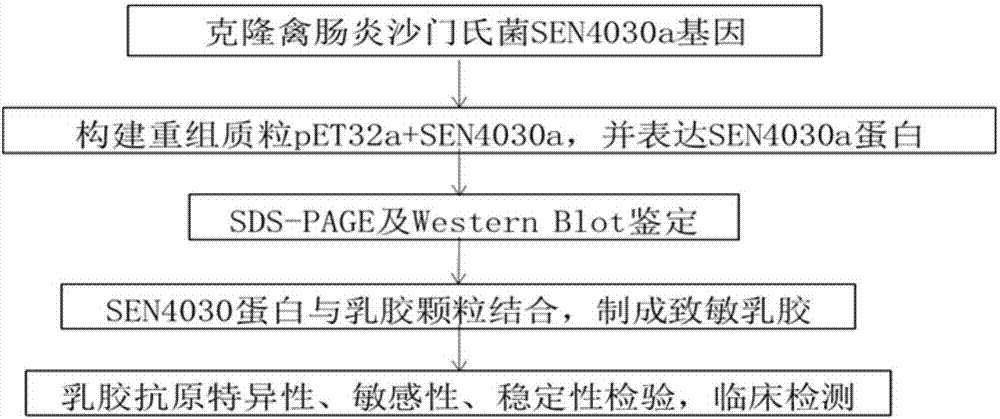
Salmonella spp antibody latex agglutination detection method
InactiveCN107085103AStrong specificityStrong characteristicBiological material analysisMicrosphereLatex particle
The invention discloses a salmonella spp antibody latex agglutination detection method belonging to the technical field of immunology. The method comprises the following steps: connecting a SEN4030a protein onto a blank latex microsphere, and preparing a sensitization latex microsphere; enabling the sensitization latex microsphere to react with a sample to be detected, and observing a agglutination result. According to the method, the SEN4030a protein is used as an antigen to prepare sensitization latex particles to detect the salmonella spp infection antibody. The SEN4030a protein has higher specificity and immunogenicity, and the prepared sensitization latex has no self-agglutination phenomenon and is high in specificity, good in repetition and stable in properties. The method provided by the invention is rapid, accurate, simple and easy; and compared with the commercialization detection antigen, the accuracy is relatively high.
Owner:CHINA AGRI UNIV
Detection card for detecting Salmonella enteritidis in tableware
InactiveCN106771183AImprove featuresIncreased sensitivityImmunoassaysQuarantineSalmonella enteritidis
The invention discloses a detection card for detecting Salmonella enteritidis in tableware. The detection card comprises a sample liquid absorption part, a baseboard, a gold marked salmonella antibody layer, a detection reaction part and a water absorption part, and the sample liquid absorption part gold marked salmonella antibody layer, the detection reaction part and the water absorption part are sequentially applied to the back lining of the baseboard from the left to the right. The detection card for detecting the residual condition of the Salmonella enteritidis in tableware has the advantages of high sensitivity and sensitivity, simplicity in operation, and no special facilities. The detection card has high specificity and sensitivity, is rapid, fast and convenient to operate in the detection process, and is suitable for being used in clinic examination, epidemiologic investigation and place quarantine. The detection card has the advantages of simple preparation method, good stability and good repeatability.
Owner:BEOSON JIANGSU FOOD SAFETY TECH CO LTD
Preparation method and application of salmonella inactivated vaccine
PendingCN112546210ANo adverse reactionGood DIVA propertiesAntibacterial agentsBiological testingAdjuvantAntiendomysial antibodies
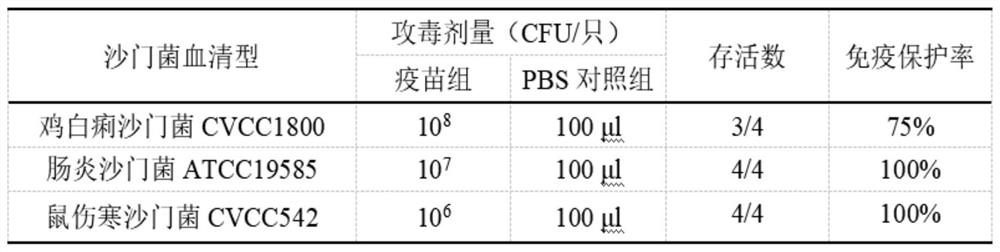
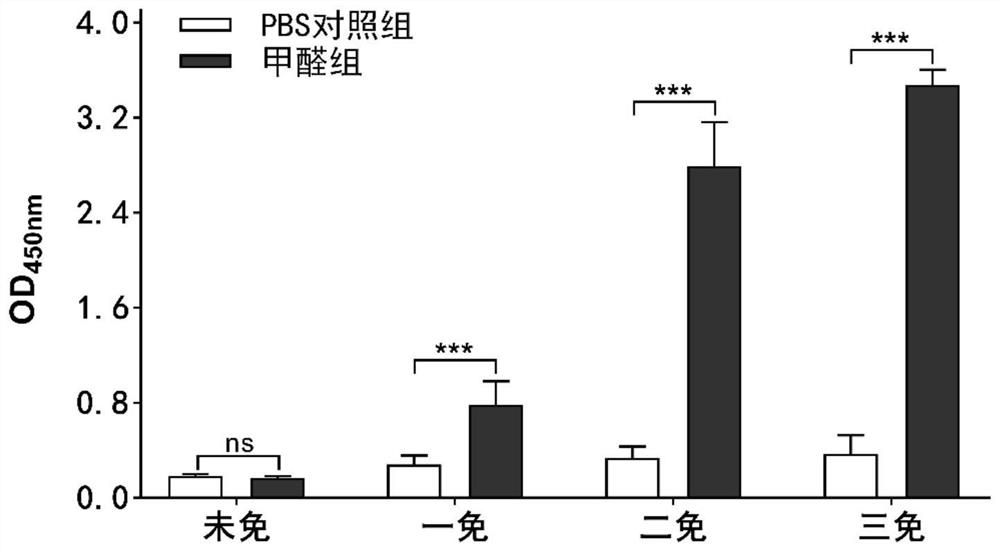
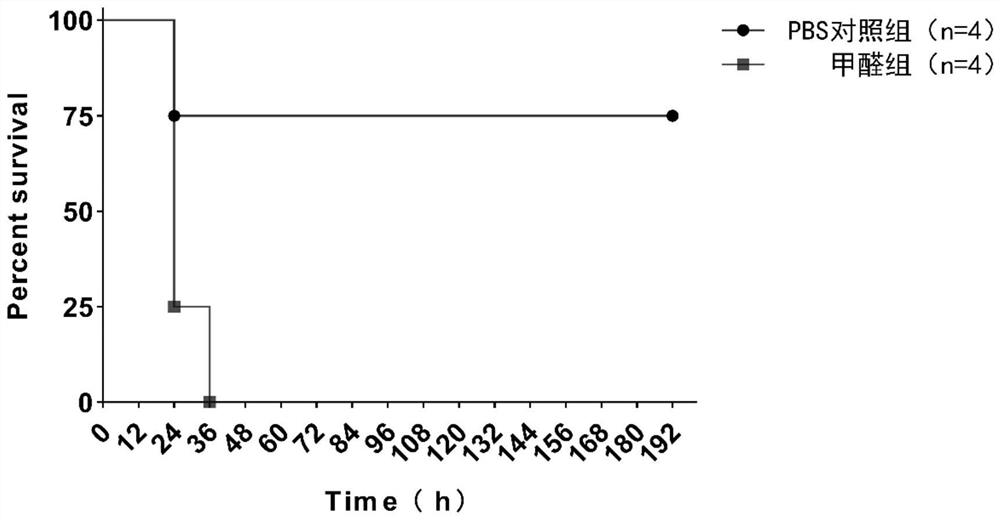
The invention discloses a preparation method and application of a salmonella pullorum gene mutant strain inactivated vaccine. The preparation method comprises the steps that a lambda-Red system is used for preparing a salmonella pullorum pagC gene deletion mutant strain delta PagC; delta PagC bacterial liquid cultured overnight is taken and inoculated into 50ml of a liquid LB culture medium according to a ratio of 1:100, and shaking culture is conducted at 37 DEG C and 180rpm until an OD value is about 0.8; a formaldehyde solution with the final concentration of 0.4% is added, shaking cultureis continuously conducted for 16 hours, centrifuging is conducted for 10 minutes at the temperature of 4 DEG C and the speed of 5000rpm, and thalli are collected, washed with a sterile PBS buffer solution, and resuspended for three times to obtain a stock solution; the dosage is adjusted, the stock solution is mixed with an adjuvant according to a ratio of 1:1, fully emulsifying is conducted, andthen the inactivated vaccine is inoculated to animals. By preparing the salmonella inactivated vaccine, no adverse reaction is caused after the vaccine strain is inoculated to a mouse, and the mouse can be protected against infection of salmonella pullorum CVCC1800, salmonella enteritidis ATCC19585 and salmonella typhimurium CVCC542. The vaccine can be used in combination with a salmonella antibody indirect ELISA detection kit based on PagC protein, differential diagnosis of salmonella artificial immunity and natural infection is achieved, and good DIVA characteristics are shown.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for simultaneously purifying and detecting avian leukosis and avian salmonellosis by utilizing one hatching egg and application of method
The invention belongs to the field of animal husbandry and veterinary and in particular relates to a method for simultaneously purifying and detecting avian leukosis and avian salmonellosis by utilizing one hatching egg and application of the method. The method comprises the following steps: feeding breeder flocks in single cages and encoding; collecting primary hatching eggs of the breeder flocks; enabling the primary hatching eggs to correspond to numbers of the hatching eggs one by one; disinfecting the surfaces of the hatching eggs and opening egg shells from hatching egg air chamber ends;sucking egg white and yolks and putting into PE (Polyethylene) tubes respectively and marking; putting egg white and yolk samples into a refrigerator and freezing and thawing for a plurality of times; taking the frozen and thawed egg white; detecting an ALV virus antigen through an avian leukosis ELISA (Enzyme Linked Immunosorbent Assay) detection method; taking the frozen and thawed yolks and adding normal saline and chloroform; after uniformly mixing on an oscillator, centrifuging and layering a solution, wherein a lower layer is an organic solvent, a middle layer is denatured lipid proteinand an upper layer is an extracted antibody; and sucking the antibody of the upper layer and detecting a salmonellosis antibody through a plate agglutination method; and eliminating chicken flock individuals with positive detection results of the ALV virus antigen and the salmonellosis antibody.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Pullorum disease antibody latex agglutination negative selection detection kit as well as preparation method and application thereof
The invention discloses a pullorum disease antibody latex agglutination negative selection detection kit, comprising a salmonella surface protein InvJ recombinant protein sensitized latex reagent and a secreted protein SopA C-terminal recombinant protein sensitized latex reagent, wherein the InvJ recombinant protein sensitized latex reagent can rapidly detect a salmonella antibody by virtue of a latex agglutination test, the SopA C-terminal recombinant protein sensitized latex reagent can detect serotype salmonella antibodies except for the pullorum disease by virtue of the latex agglutination test, and the combination of the two reagents is used for negative selection of a pullorum disease antibody positive blood or serum sample. The invention also discloses a preparation method of salmonella surface protein InvJ recombinant protein and secreted protein SopA C-terminal recombinant protein sensitized latex reagents. The pullorum disease antibody latex agglutination negative selection detection kit disclosed by the invention has the advantages of stable antigen, strong specificity and high sensitivity, and solves the problem of antigen instability caused by potential variability and changed culture conditions of an existing pullorum disease agglutination antigen production strain.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
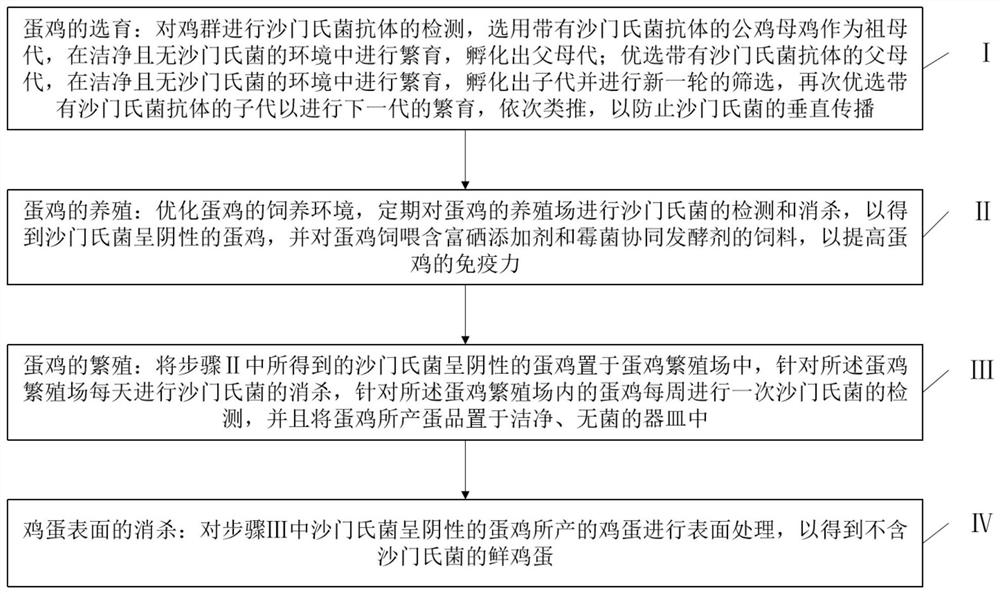
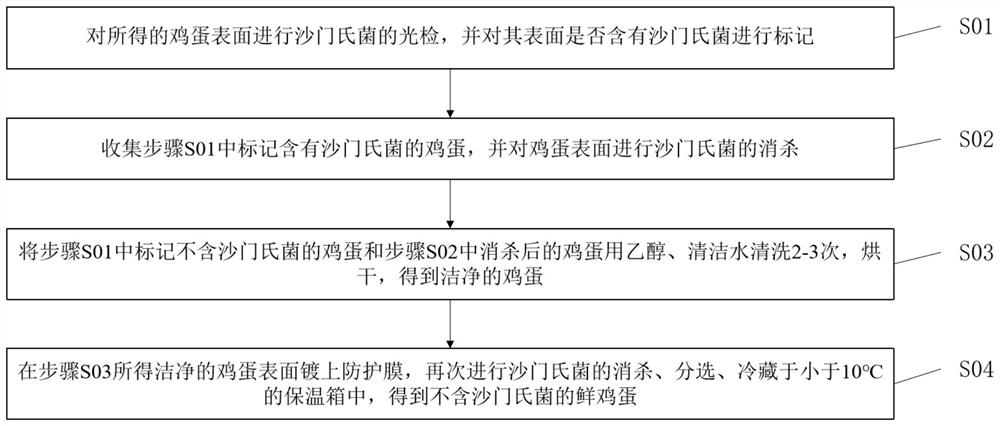
Sterile egg production process for salmonella
PendingCN114145264AImprove removal efficiencyImprove immunityAnimal feeding stuffAccessory food factorsBiotechnologyAnimal science
The invention relates to the technical field of sterile egg production, in particular to a sterile egg production process aiming at salmonella, which comprises the following steps: firstly, carrying out salmonella antibody-containing breeding on female chickens, then optimizing the breeding environment of laying hens, namely, breeding the laying hens in different houses, and regularly detecting and killing the breeding farm of the laying hens; laying hens are fed with feed containing a selenium-rich additive and a mould synergistic leavening agent, the laying hens with negative salmonella are obtained, then a breeding farm of the laying hens is subjected to high-intensity sterilization to prevent vertical propagation and horizontal propagation of salmonella, the laid eggs are placed in a clean and sterile vessel, then the surfaces of the eggs are treated, and the laying hens with the negative salmonella are obtained. And finally obtaining the salmonella-free fresh eggs. According to the method, the detection rate of salmonella in the sterile eggs is remarkably reduced, the operation is simple, the breeding cost is saved, and commercial application is facilitated.
Owner:安徽鲜森绿色食品有限公司
ELISA kit for detecting salmonella antibody, detection method and application thereof
The invention relates to an ELISA kit for detecting salmonella antibody, a detection method and application thereof, belonging to the technical field of microbial immunology. The ELISA kit comprises antigen protein or antigen protein solution or a coated plate coated with the antigen protein solution, an enzyme-labeled antibody, color developing solution, stop solution, positive serum and negativeserum. The kit can quickly, specifically and sensitively detect the salmonella antibody in the serum, and the sensitivity of detecting the serum antibody is 1: 1000; and the kit has simple and convenient operation, low cost, easy observation of reaction result, high sensitivity and strong specificity, and is applicable to the technical fields of salmonella monitoring, epidemiological investigation, clinical sample detection and the like.
Owner:SOUTH CHINA AGRI UNIV
Anti-salmonella antibodies and uses thereof
ActiveUS20180030120A1Reduce presenceAntibacterial agentsImmunoglobulins against bacteriaAntibody fragmentsSingle-domain antibody
The present disclosure provides anti-Salmonella antibodies or antibody fragments, such as camelid single domain antibodies (VHHs), along with associated nucleic acids, host cells and phages. Methods of reducing the presence of Salmonella in an animal or an animal environment, methods and formulations for treating Salmonella infection, and methods of detecting Salmonella are also described.
Owner:ABCELEX TECH INC
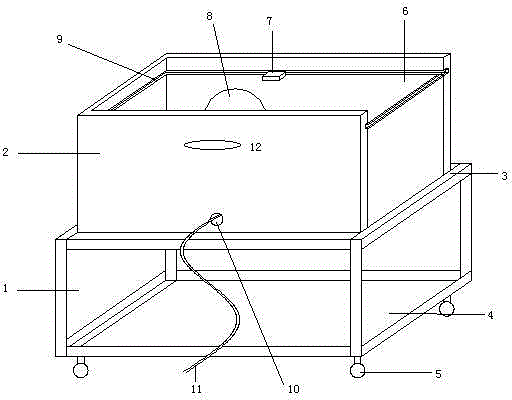
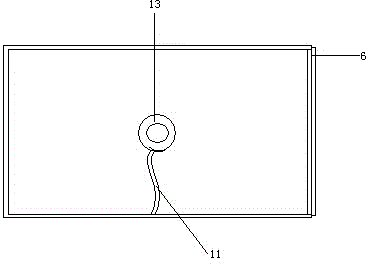
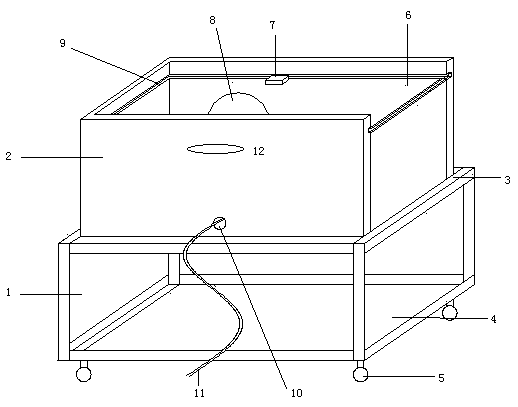
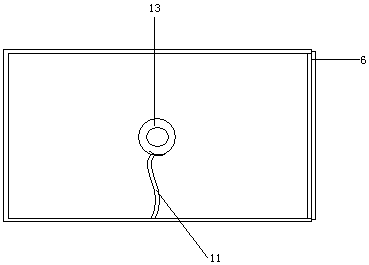
Cart for detecting salmonella antibody in henhouse
ActiveCN104477220AImprove accuracySmooth and movableHand carts with multiple axesMaterial analysisEngineeringSalmonella antibody
The invention discloses a cart for detecting a salmonella antibody in a henhouse. The cart comprises a frame and a box body, wherein the frame is provided with a box body supporting plate and wheels; the box body supporting plate is arranged at the upper part of the frame and is used for supporting the box body; the wheels are arranged below the frame; the box body is a box-shaped component; the top surface of the box body is transparent; a lamp holder is mounted in the internal space of the box body and is used for holding a bulb. An incandescent bulb or an energy-saving lamp can be mounted on the lamp holder according to the seasons, an appropriate environment temperature is provided for a detection material placed on the top surface, a light source is stable, and observation is facilitated.
Owner:ANHUI RONGDA POULTRY DEV
Pullorum antibody latex agglutination negative screening kit, preparation method and application
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Method for sensitively, simply and conveniently detecting bacteria
InactiveCN102841198BSimple stepsEasy to detectFluorescence/phosphorescenceSalmonella kielFluorescence microscope
The invention relates to a method for detecting bacteria. The method comprises the steps as follows: coupling magnetic nanospheres and fluorescent nanospheres with mouse typhus salmonella antibodies so as to obtain mouse typhus salmonella-targeted immunologic magnetic spheres and immunologic fluorescent spheres, and adding the mouse typhus salmonella-targeted immunologic magnetic spheres and immunologic fluorescent spheres into a detection system so as to realize magnetic capture and fluorescent labeling to the mouse typhus salmonella simultaneously. About 10 CFU / mL of the mouse typhus salmonella can be detected through observation of a fluorescent microscope; and a good quantitative relation is obtained through detection of a fluorescence spectrophotometer, the linear range is 105-107 CFU / mL, and R2 is equal to 0.9994. The whole detection process is very simple, high in sensitivity and strong in specificity, and can be finished detection within 1.5 h.
Owner:WUHAN UNIV
An elisa kit for detecting antibodies against Salmonella pullorum
ActiveCN103995126BReduce stressImprove featuresBiological material analysisBiological testingAntigenElisa kit
An ELISA kit for detecting a Salmonella pullorum antibody is established by screening Salmonella pullorum dominant antigen GroEL through an immunoprecipitation technology, expressing GroEL recombinant protein through utilizing a prokaryotic expression vector, and utilizing an antigen protein. The kit can reduce the response of chicken in the detection process of the Salmonella pullorum antibody, and can improve the detection specificity and the sensitivity.
Owner:WENS FOODSTUFF GRP CO LTD
Salmonella and bacteria total number rapid synchronous multiple detection method and kit
PendingCN112553292AGood linear relationshipImprove accuracyMicrobiological testing/measurementBiological material analysisSalmonella danSalmonella antibody
The invention discloses a method and detection kit for rapidly and synchronously detecting the total number of salmonella and bacteria in a sample in multiple times. A red fluorescent probe capable ofmarking all bacteria is used for marking the total number of bacteria to distinguish the bacteria and other background particles in the sample, and meanwhile, by a green fluorescent probe, a salmonella antibody cross-linked through a chemical group is used for distinguishing salmonella and non-salmonella in the sample; and red and green fluorescence signals are simultaneously counted through a flow analyzer, so that the synchronous detection of the total number of salmonella and bacteria is realized. The method can be used for simultaneously detecting the total number of salmonella and bacteria, is simple and convenient to operate and short in consumed time, and can be used for detecting most samples within 0.5 hour.
Owner:NAT INST OF METROLOGY CHINA
Salmonella cleia detection kit and detection method
InactiveCN103513036BHigh detection sensitivityHigh sensitivityImmunoassaysMicrotiter plateImmuno detection
The invention discloses a CLEIA detection kit and a detection method of salmonella. The kit includes a salmonella antibody, a horseradish peroxidase labeled salmonella antibody and a chemiluminescent solution containing p-lodophenol, luminol and hydrogen peroxide. The corresponding detection method uses the salmonella antibody coated in a 96 well microtiter plate as a capture antibody, the horseradish peroxidase labeled salmonella antibody as an antibody for detection, and luminol-H2O2 with a p-iodophenol reinforcing agent as the chemiluminescent solution, so as to establish a double antibody sandwich chemiluminescent enzyme immunoassay method for detecting content of salmonella. The method provided by the invention has the advantages of high sensitivity, good selectivity, easy operation, fast detection and low cost, can realize large-scale online detection of salmonella, and is suitable for extensive application in the fields of food safety and environmental monitoring, etc.
Owner:徐静 +5
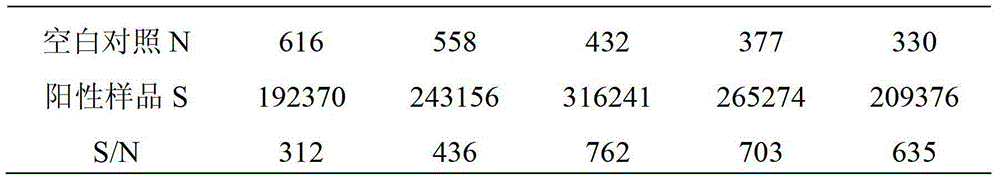
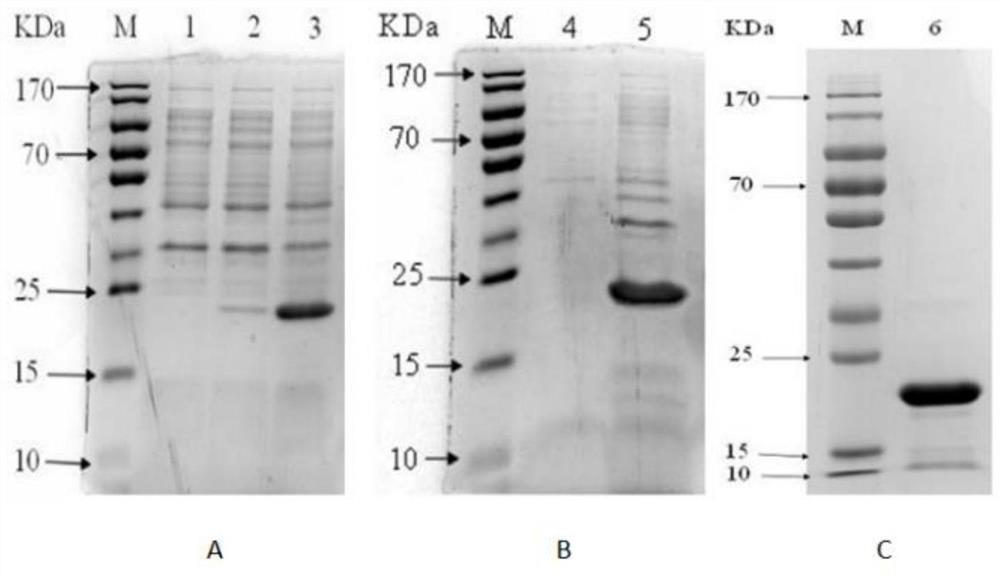
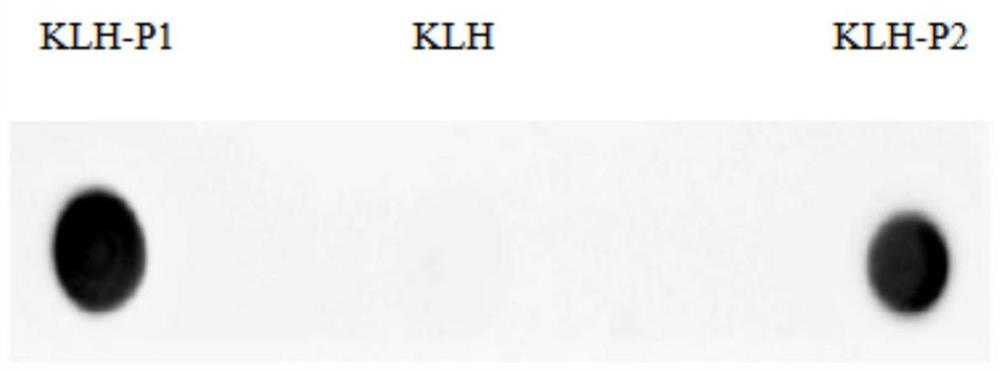
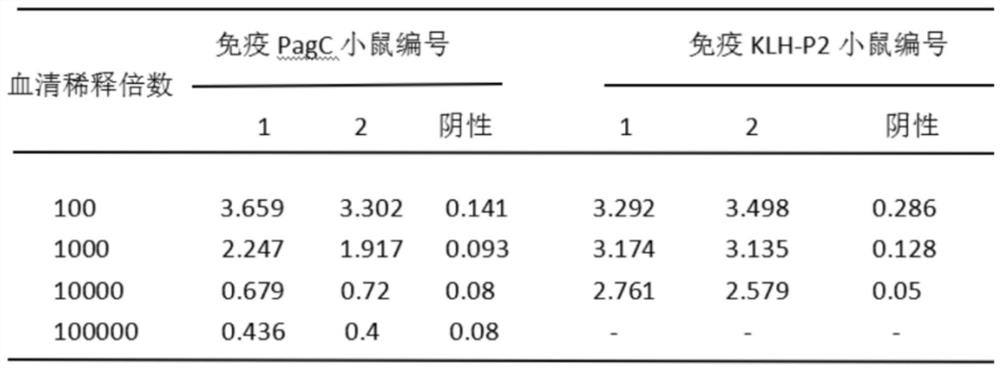
Preparation method and screening method of salmonella PagC protein monoclonal antibody
The invention discloses a preparation method and a screening method of a salmonella PagC protein monoclonal antibody. The preparation method comprises the following steps of immunizing Balb / c mice byusing purified PagC protein, and obtaining six hybridoma cells capable of producing an anti-PagC protein monoclonal antibody according to a hybridoma cell preparation technology; and synthesizing intergeneric specific amino acid sequences DRQASGSVEPEGIH(P1) and FKEHSTQDGDSFNKISSRKTGFA(P2) containing linear epitopes as screening antigens to obtain a monoclonal antibody cell strain J of which the antigen epitopes are on a linear P1 sequence, wherein the other five monoclonal antibody epitopes are not on linear P1 and P2 amino acid sequences. Western-blotting results show that mAb J can react with purified PagC protein, wild type pullorum disease and mouse typhoid fever PagC protein and does not react with other enterobacteriaceae PagC protein, and it is shown that the screened mAb J epitopeP1 is conservative in salmonella and is intergeneric specific. By preparing the monoclonal antibody, a salmonella antibody blocking ELISA detection method can be established, and the monoclonal antibody has the characteristics of good specificity and high sensitivity and has wide popularization potential.
Owner:NANJING AGRICULTURAL UNIVERSITY
A kit for detecting antibody to Salmonella swine
ActiveCN109425735BEasy to operateReduce use costDepsipeptidesMaterial analysisProtein s antigenSerum dilution
Owner:CHINA AGRI UNIV
Construction method for engineered strain capable of preparing series of specific salmonella diagnostic serum
InactiveCN103484416AMethod is feasibleHigh feasibilityBacteriaMicroorganism based processesSalmonella concordImmunologic function
The invention discloses a construction method for an engineered strain capable of preparing a series of specific salmonella diagnostic serum. The method utilizes a modern molecular biological technology to modify a traditional polyclonal antibody preparation system so as to construct host bacteria lack of surface flagellar antigen synthesis genes; the surface flagellar antigen synthesis genes of different pathogene microbes are cloned on suitable expression vectors, and then the vectors are transferred to the host bacteria lack of the antigen genes, so as to obtain a series of engineered strains which have the same genetic background, and contain specific surface flagellar antigen genes of the different pathogene microbes. Due to the adoption of the technologies, a library containing a plurality of specific salmonella antibodies can be constructed. The antibody library can supplement current antiserum types, and meanwhile can lay foundation for the application of other immunology technologies.
Owner:NANKAI UNIV
A kind of ELISA kit for detecting Salmonella antibody and its detection method and application
The invention relates to an ELISA kit for detecting Salmonella antibody, a detection method and application thereof, and belongs to the technical field of microbial immunology. It comprises antigenic protein or antigenic protein solution or a coated plate coated with said antigenic protein solution, enzyme-labeled antibody, color development solution, stop solution, positive serum and negative serum. The kit can quickly, specifically and sensitively detect Salmonella antibodies in serum, and the sensitivity of detecting serum antibodies is 1:1000; the kit is easy to operate, low in cost, easy to observe the reaction results, high in sensitivity and strong in specificity, and is suitable for Salmonella monitoring, epidemiological investigation and detection of clinical samples and other technical fields.
Owner:SOUTH CHINA AGRI UNIV
Anti-salmonella antibodies and uses thereof
InactiveUS10421806B2Reduce presenceAntibacterial agentsImmunoglobulins against bacteriaAntiendomysial antibodiesAntibody fragments
The present disclosure provides anti-Salmonella antibodies or antibody fragments, such as camelid single domain antibodies (VHHs), along with associated nucleic acids, host cells and phages. Methods of reducing the presence of Salmonella in an animal or an animal environment, methods and formulations for treating Salmonella infection, and methods of detecting Salmonella are also described.
Owner:ABCELEX TECH INC
A trolley for salmonella antibody detection in a chicken coop
ActiveCN104477220BEasy to moveImprove accuracyHand carts with multiple axesMaterial analysisEngineeringSalmonella antibody
Owner:ANHUI RONGDA POULTRY DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com