Synthesis method of ortho-benzyl diphosphine compound
A synthesis method and technology of phosphine compounds, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, compounds of Group 5/15 elements of the periodic table, etc., can solve low yield and unfavorable industrialization Production and other issues, to achieve high yield, improve safety and controllability, mild and controllable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] One embodiment of the present invention provides a method for synthesizing an ortho-benzyl bisphosphine compound, comprising the following steps:
[0061] In an organic solvent, α,α'-dihalogenated o-xylene and dihydrocarbyl phosphine are subjected to a substitution reaction under the action of a metal salt catalyst, a ligand and a basic compound to obtain an ortho-benzyl bisphosphine compound.
[0062] Wherein, the metal in the metal salt catalyst is a transition metal, and the ligand can coordinate with the metal salt catalyst.
[0063] Dihydrocarbylphosphine is , R is independently selected from: alkyl, aryl or cycloalkyl.
[0064] In some embodiments, metal salt catalysts and ligands can also be added in a pre-coordinated form.
[0065] The structural formula of α,α'-dihalogenated o-xylene is as follows:
[0066]
[0067] Wherein, X is a halogen, further X is independently selected from Cl or Br.
[0068] The reaction formula of this synthetic method is as fo...
Embodiment 1
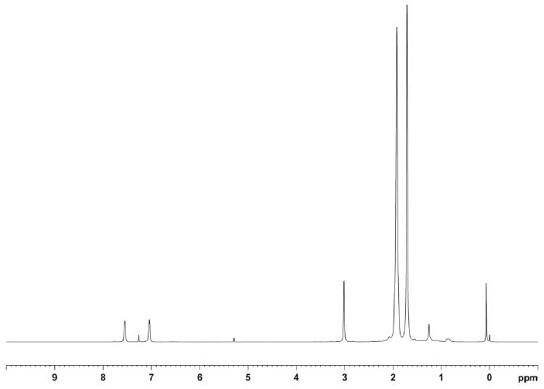
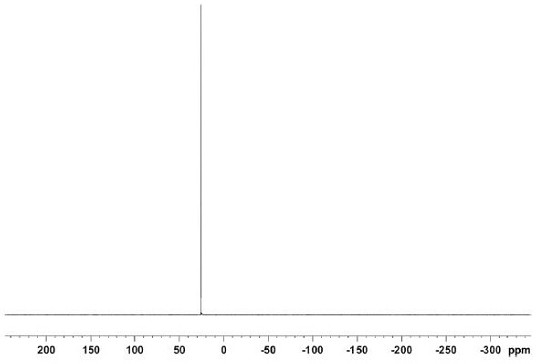
[0088] This example is a synthesis method of ortho-benzyl bisphosphine compounds, specifically the synthesis steps of bis(diamantylphosphine)-o-xylene. The whole process is carried out in an anhydrous and oxygen-free environment, and the steps are as follows:
[0089] Under Ar atmosphere, add o-dibromobenzyl (300g, 1eq) to 2L toluene solution to dissolve it completely, then add diadamantyl phosphine hydrogen (687g, 2eq), add metal salt catalyst Pd(OAc) 2 (7.6g, 3%eq), ligand DIPPF (15.7g, 3.3%eq) and tBuONa (279g, 2.5eq), heated to reflux, the solid gradually dissolved, reacted for 4h, TLC monitored the reaction was complete, stopped the reaction and cooled to room temperature .
[0090] Remove the solvent by distillation under reduced pressure, then add dichloromethane to dissolve the solid completely (1L), wash with water (1L×3 times), wash with saturated sodium chloride solution (1L×3 times), dry over anhydrous sodium sulfate, filter, and normal pressure The solvent was di...
Embodiment 2
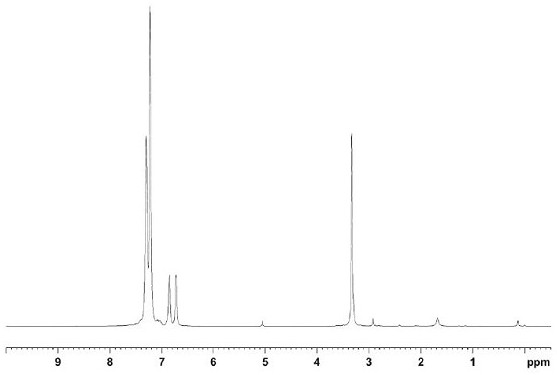
[0092] This embodiment is a synthesis method of ortho-benzyl bisphosphine compounds, specifically the synthesis steps of bis(diphenylphosphine)-o-xylene. The whole process is carried out in an anhydrous and oxygen-free environment, and the steps are as follows:
[0093] Under Ar atmosphere, add o-dibromobenzyl (300g, 1eq) to 2L toluene solution to dissolve it completely, then add diphenylphosphine hydrogen (423g, 2eq), add metal salt catalyst Pd(OAc) 2 (7.6g, 3%eq), ligand DIPPF (15.7g, 3.3%eq) and tBuONa (279g, 2.5eq), heated to reflux, the solid gradually dissolved, reacted for 4h, TLC monitored the reaction was complete, stopped the reaction and cooled to room temperature .
[0094] Remove the solvent by distillation under reduced pressure, then add dichloromethane to dissolve the solid completely (1L), wash with water (1L×3 times), wash with saturated sodium chloride solution (1L×3 times), dry over anhydrous sodium sulfate, filter, and normal pressure The solvent was dist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com