A kind of biologically active peptide rlafiahpklg and its preparation method and application
A technology of biologically active peptides and sequences, applied in the field of protein, to achieve good anti-inflammatory activity, improved ability, and improved quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Artificial synthesis of bioactive peptide RLAFIAHPKLG
[0031] 1. Synthesis of Bioactive Peptides
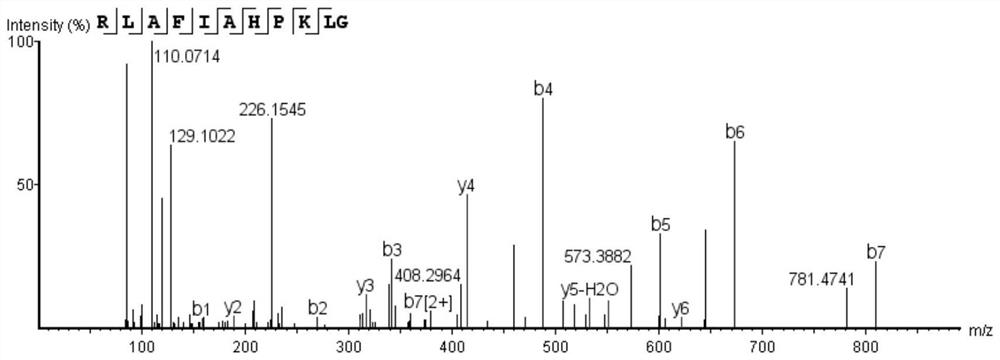
[0032] Synthetic bioactive peptide RLAFIAHPKLG.
[0033] 2. Confirmation of Bioactive Peptides
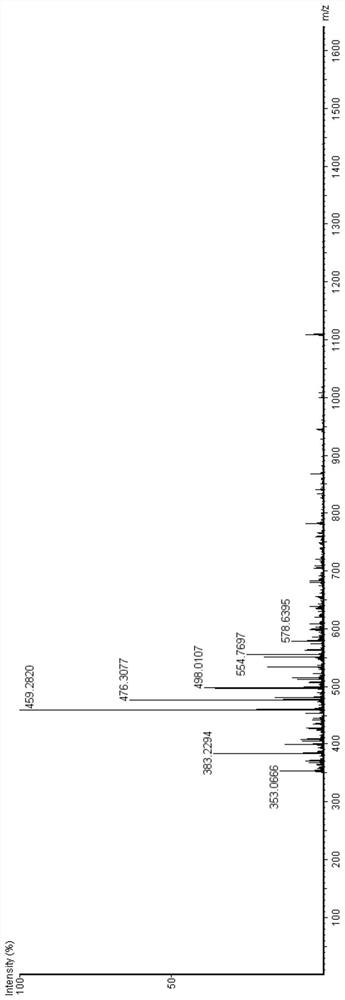
[0034] 1) UPLC analysis
[0035] The UPLC conditions are as follows:
[0036] Instrument: Waters ACQUITY UPLC ultra-high performance liquid phase, electrospray, quadrupole, time-of-flight mass spectrometer
[0037] Column Specifications: BEH C18 Column
[0038] Flow rate: 0.4mL / min
[0039] Temperature: 50℃
[0040] UV detection wavelength: 210nm
[0041] Injection volume: 2μL
[0042] Gradient conditions: solution A: water containing 0.1% formic acid (v / v), solution B: acetonitrile containing 0.1% formic acid (v / v)
[0043] Time(min) %A %B
0 95.0 5.0
1.50 80.0 20.0
3.50 60.0 40.0
5.00 40.0 60.0
7.00 15.0 85.0
8.00 0.0 100.0
11.00 0.0 100.0
11.50 95.0 5.0
13.00 95.0 5.0
[0044] 2) Mass spectrometry
[0045] The mass spectrometry conditio...
Embodiment 2
[0059] Example 2 Immunoactivity test of biologically active peptides
[0060] 1. In vitro lymphocyte proliferation ability test of bioactive peptide RLAFIAHPKLG (MTT method)
[0061] 1. Experimental materials and instruments:
[0062] Reagents and materials: experimental animals balb / c mice (male, 6-8 weeks old, Animal Experiment Center, School of Agriculture and Biology, Shanghai Jiaotong University); mouse spleen lymphocyte-derived bioactive peptide RLAFIAHPKLG obtained in Example 1; mouse lymphocytes Cell extract (purchased from Soleobor Company); RPMI1640 medium (purchased from GIBCO Company); 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide ( MTT, purchased from Amresco Company); Concanavalin (ConA, purchased from Sigma Company); Bovine Serum Albumin (BSA, purchased from Genebase Company); Pepsin (purchased from Sigma Company); Pancreatin (Corolase PP, purchased from Sigma Company) from AB Company).
[0063] Equipment: LRH-250F biochemical incubator, Shanghai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ion source temperature | aaaaa | aaaaa |
| collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com