Nitrile hydratase mutant and application thereof
A technology of nitrile hydratase and nitrile hydratase, which is applied in the field of bioengineering and can solve the problems of limited thermal stability and low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Plasmid construction of nitrile hydratase mutants
[0033] Using the wild-type plasmid pET24a(+)-Cal.t WT (Zhang Sailan, Li Ting, Cheng Zhongyi et al. Study on heterologous expression and catalytic process of a new heat-resistant nitrile hydratase[J]. Food and Fermentation Industry, 2020, Volume 46 (14): pET24a (+)-Cal.t NHase) in 108-113 is a template, the mutation sites L48D and L48H are designed on the primers, and the plasmids with mutated base sequences are amplified by PCR. The primer sequences are shown in Table 1, and the amplification system is shown in Table 2.
[0034] The PCR amplification reaction conditions were pre-denaturation at 95°C for 3min, denaturation at 98°C for 15s, annealing at 55°C for 30s, extension at 72°C for 1min45s, and extension at 72°C for 5min, a total of 30 cycles. The PCR product was digested with DpnI digestive enzyme for 2-3h, transformed into E.coli DH5α, spread on LB medium plate containing 50mg / L kanamycin, and incuba...
Embodiment 2
[0042] Example 2: Expression and purification of wild enzyme WT and each mutant
[0043] Step 1: Transform the wild-type pET24a(+)-Cal.t WT of Cal.t NHase obtained in Example 1 and the reconstituted plasmids pET24a(+)-L48D and pET24a(+)-L48H into E.coli BL21(DE3 ), pick a single colony into 5mL LB medium, and culture at 37°C and 200rpm for 7-8h. The seed solution was transferred to 100mL 2×YT medium according to the inoculation amount of 1% (v / v), cultured at 37°C and 200rpm until the OD600 reached 0.6-0.8, and the final concentration was 0.4mM isopropylthiosemi Lactose (IPTG) and 0.1g / L CoCl 2 ·6H 2 O, change the culture temperature to 24°C, and induce expression for 12-16 hours.
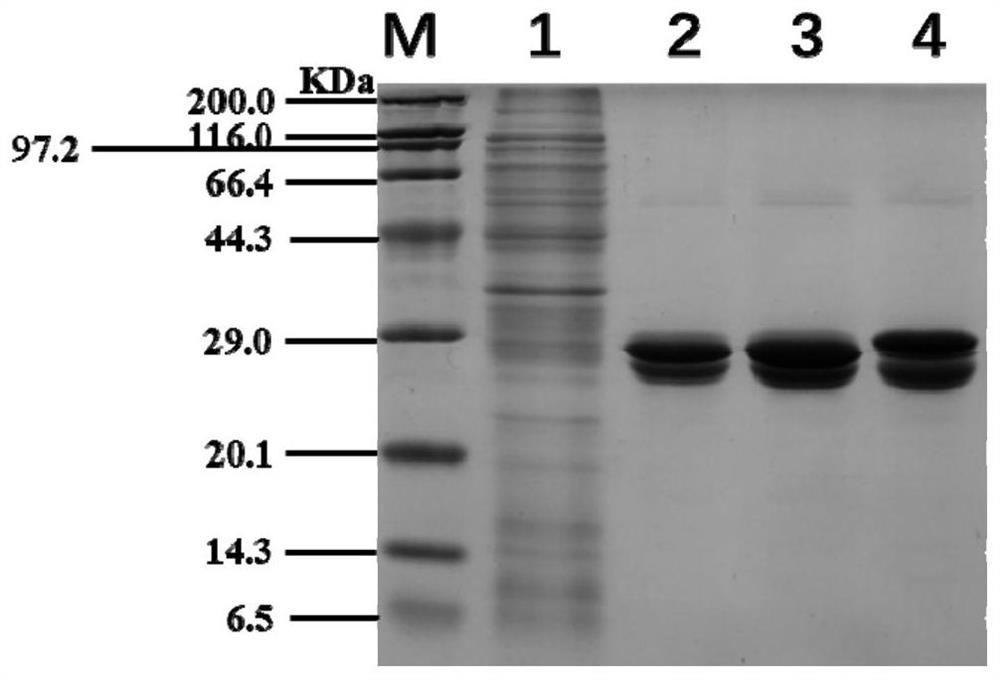
[0044]Step 2: The wild-type WT and its two mutants were purified by affinity chromatography, and the purification column was a StrepTrap HP 1mL column from GE. The bacterial cells were collected by centrifugation at 10,000 rpm for 3 min, resuspended with 20 mL of binding buffer, and ultrasonica...
Embodiment 3
[0045] Example 3: Detection of wild type and mutant catalytic efficiency of Cal.t NHase
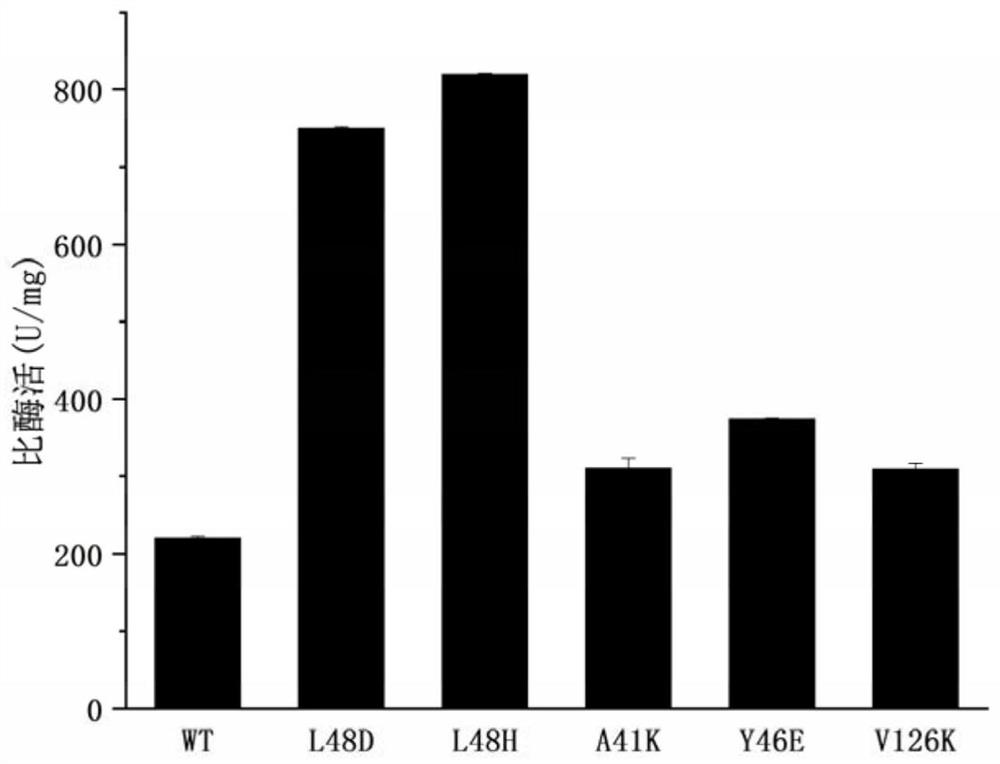
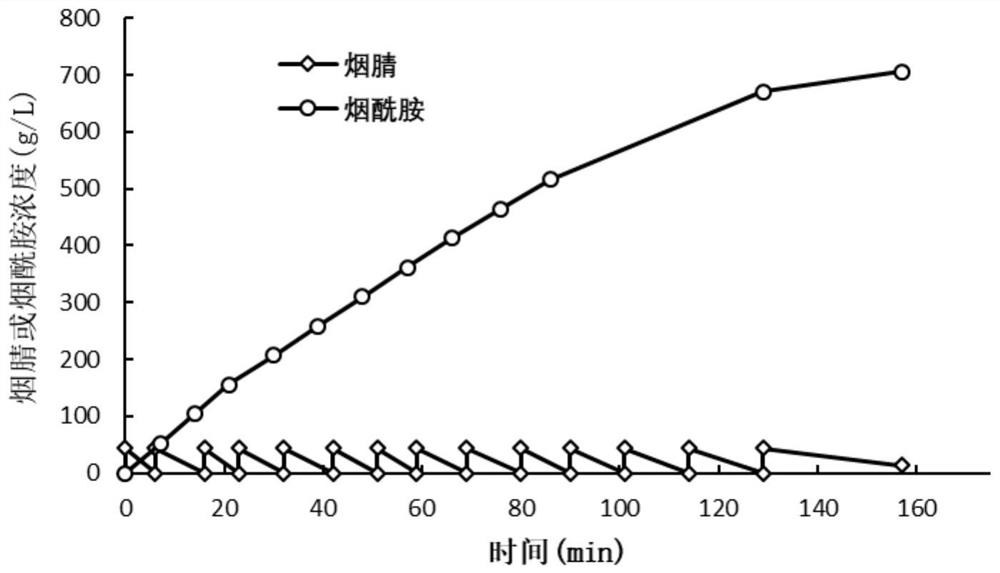
[0046] Dilute the pure enzyme concentration of WT and its mutants to 0.5 mg / mL with 10 mM KPB (pH 7.4) solution, take 10 μL into a 1.5 mL centrifuge tube, and place it on a metal bath at 25 °C. Add 490 μL of substrate (200 mM nicotinonitrile solution) to the centrifuge tube, vortex and mix thoroughly, react at 25° C. for 10 min, and then add 500 μL of pure acetonitrile solution to terminate. The reaction solution was diluted with pure acetonitrile solution to an appropriate multiple, and passed through a 0.22 μm filter membrane. Liquid phase detection method: the mobile phase composition is acetonitrile: water = 1:2 (v / v), the flow rate is 0.6mL / min, the detection wavelength is 215nm, the column temperature is 40°C, and the amount of nicotinamide produced in the reaction system is determined . The calculation results of specific enzyme activities of WT and mutants are as follows: figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com