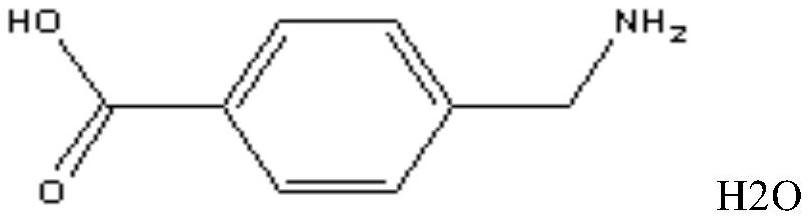
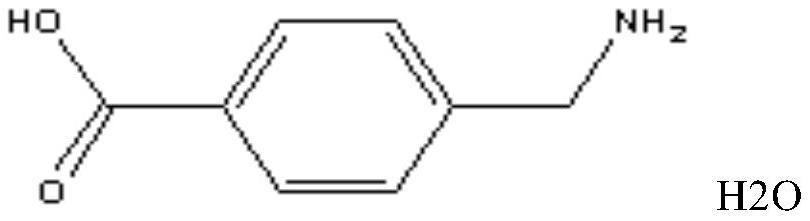
Freeze-dried powder injection of aminomethylbenzoic acid for injection
A technology of freeze-dried powder for injection and aminotoluic acid, which is applied in the field of freeze-dried powder of aminotoluic acid for injection, and can solve the problems of inapplicable powder for injection and inapplicable powder for injection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0112] Preparation Example 1, Preparation of Powder Injection Containing Aminotoluic Acid
[0113] formula:
[0114] Aminomethylbenzoic acid 100mg, Mannitol 95 mg, Dextran-20 5 mg, pH regulator to pH4.0, Water for Injection Appropriate amount, add to 4ml.
[0115] Preparation:
[0116] (1) Take the principal agent and auxiliary materials of the prescription quantity, place in a stainless steel bucket, add about 80% of the prescription quantity of water for injection, make each component dissolve, then add 0.1% (w / v) gac by the volume of the solution, Stir for 30 minutes, filter and decarburize, and add water for injection to nearly the full amount of the prescription.
[0117] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (this specified value is the value of the measured pH value of the freeze-dried gained dry powder diluted with water for inje...
preparation example 2
[0121] Preparation Example 2, Preparation of Powder Injection Containing Aminotoluic Acid
[0122] formula:
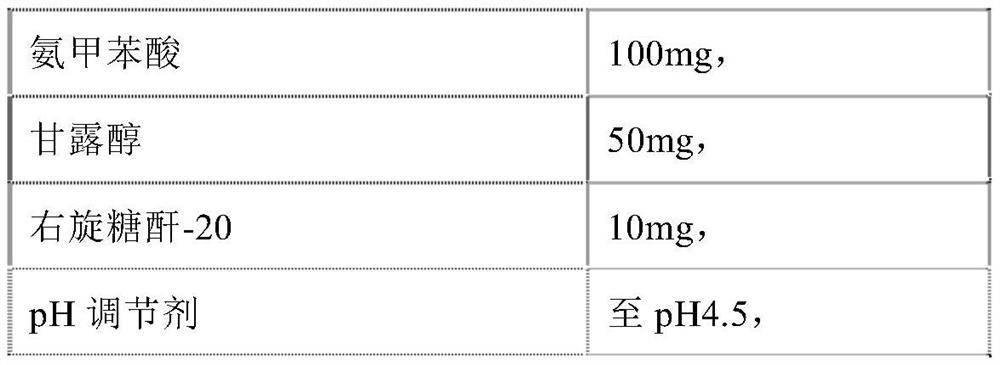
[0123]
[0124]
[0125] Preparation:
[0126] (1) Take the principal agent and auxiliary materials of the prescription quantity, place in a stainless steel bucket, add about 80% of the prescription quantity of water for injection, make each component dissolve, then add 0.1% (w / v) gac by the volume of the solution, Stir for 30 minutes, filter and decarburize, and add water for injection to nearly the full amount of the prescription.
[0127] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (this specified value is the value of the measured pH value of the freeze-dried gained dry powder diluted with water for injection into a solution containing 10 mg / ml of the active ingredient, The same below), and then add water for injection to the full amount of the prescription.
[0128]...
preparation example 3
[0131] Preparation Example 3, Preparation of Powder Injection Containing Aminotoluic Acid
[0132] formula:
[0133] Aminomethylbenzoic acid 100mg, Mannitol 150mg, Dextran-40 2 mg, pH regulator to pH3.5, Water for Injection Appropriate amount, add to 5ml.
[0134] Preparation:
[0135] (1) Take the principal agent and auxiliary materials of the prescription quantity, place in a stainless steel bucket, add about 80% of the prescription quantity of water for injection, make each component dissolve, then add 0.1% (w / v) gac by the volume of the solution, Stir for 30 minutes, filter and decarburize, and add water for injection to nearly the full amount of the prescription.
[0136] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (this specified value is the value of the measured pH value of the freeze-dried gained dry powder diluted with water for injec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com