Catalyst component for olefin polymerization, preparation method of catalyst component, catalyst for olefin polymerization and polymerization method
A technology of olefin polymerization and catalyst, which is applied in the field of olefin polymerization, can solve the problems of fast decay of catalytic activity, narrow molecular weight distribution, poor hydrogenation sensitivity of catalyst, etc., and achieve the effect of wide molecular weight distribution, widening molecular weight distribution and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
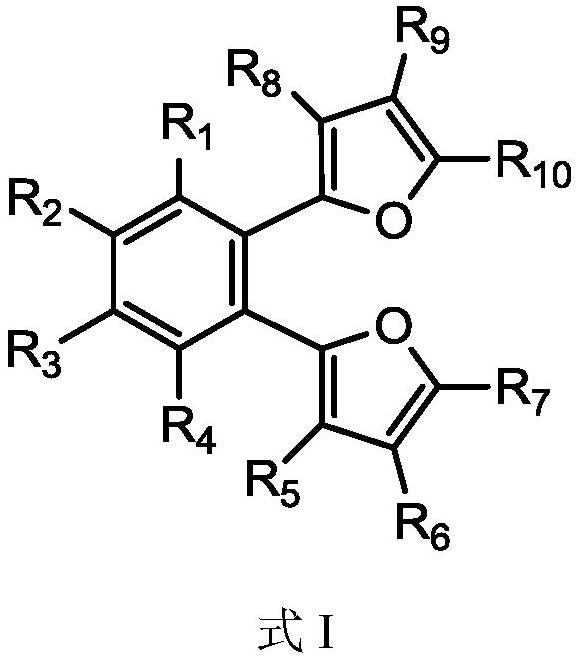
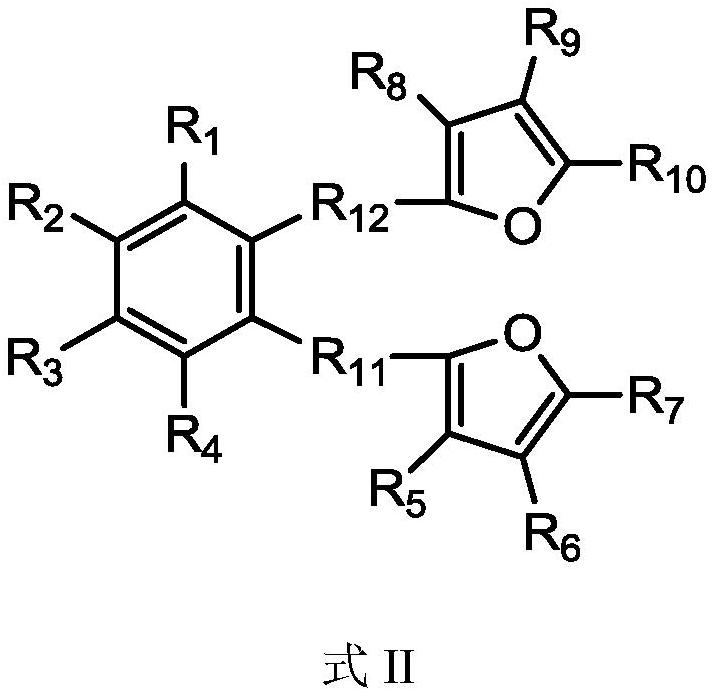
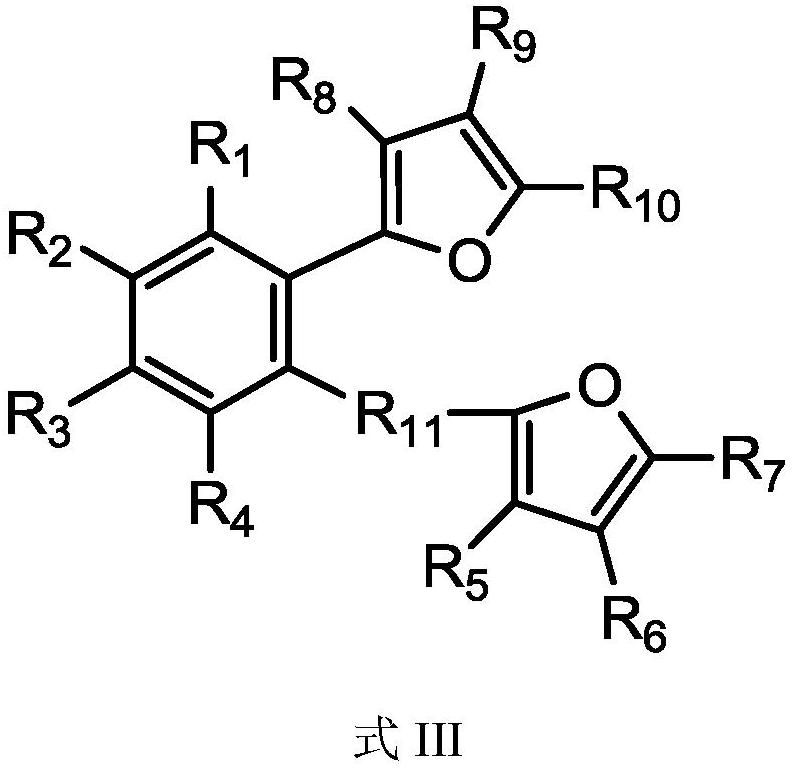
[0041] Under a nitrogen atmosphere, add 74 mmol of furan to 20 mL of tetrahydrofuran solution, cool down to -78°C, slowly add 44 mmol of n-butyllithium, stir and slowly raise the temperature, and react for 4 hours after rising to room temperature; then cool the solution to -78°C, 34 mmol o-dibromobenzene was added dropwise, stirred and slowly raised to room temperature for 12 h. Pour the reaction product into ice water, extract the organic phase with ethyl acetate, then dry the organic phase with magnesium sulfate to remove water, filter, the resulting solution is concentrated under reduced pressure and separated by column chromatography, the developer is ethyl acetate / petroleum Ether (1:50), the oily product was obtained, and the waxy product 1,2-bis(2-furan)benzene (abbreviated as internal electron donor a) was obtained after removing the solvent, with a yield of 47%. 1 H-NMR (CDCl3): δ=7.86 (4H, m, C=CH), 7.47 (2H, m, C=CH), 7.07 (2H, d, C=CH), 6.68 (2H, m, C=CH) CH).
[...
Embodiment 2
[0044] Under nitrogen atmosphere, add 70mmol of furan into 20mL of tetrahydrofuran solution, cool down to -78°C, slowly add 40mmol of n-butyllithium, stir and slowly raise the temperature, rise to room temperature and react for 4h; then cool the solution to -78°C, 20 mL of 30 mmol of 2,3-dibromonaphthalene in tetrahydrofuran was added dropwise, stirred and slowly raised to room temperature for 12 h. Pour the reaction product into ice water, extract the organic phase with ethyl acetate, dry the organic phase with magnesium sulfate to remove water, filter, and concentrate the obtained solution under reduced pressure, then recrystallize in ethanol to obtain the solid product 2,3- Bis(2-furan)naphthalene (abbreviated as internal electron donor b), the yield is 55%. 1 H-NMR (CDCl3): δ = 8.40 (2H, s, C = CH), 8.00 (2H, d, C = CH), 7.86 (2H, d, C = CH), 7.59 (2H, m, C = CH), 7.07 (2H,d,C=CH), 6.68 (2H,m,C=CH).
[0045]
Embodiment 3
[0047] Under a nitrogen atmosphere, add 77 mmol of 2-methyl-furan into 20 mL of tetrahydrofuran solution, cool down to -78°C, slowly add 45 mmol of n-butyllithium, stir and slowly raise the temperature, and react for 4 hours after rising to room temperature; Cool down to -78°C, add 35 mmol of 1,2-(dibromomethyl)benzene in 20 mL of tetrahydrofuran solution dropwise, stir and slowly rise to room temperature to react for 12 h. Pour the reaction product into ice water, extract the organic phase with ethyl acetate, dry the organic phase with magnesium sulfate to remove water, filter, and concentrate the obtained solution under reduced pressure, then recrystallize in ethanol to obtain the solid product 1,2- Bis(5-methyl-2-methylene-furan)benzene (abbreviated as internal electron donor c), yield 51%. 1 H-NMR (CDCl3): δ=7.14 (2H, m, C=CH), 6.98 (2H, m, C=CH), 5.96 (4H, d, C=CH), 3.66 (4H, s, CH 2 ),2.30(6H,s,CH 3 ).
[0048]
[0049] (2) Preparation of catalyst
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com